US Pharm. 2016;41(11):HS20-HS26.
ABSTRACT: Delusions and hallucinations in patients with Parkinson’s disease, a condition known as Parkinson’s disease psychosis (PDP), have historically been treated with clozapine and quetiapine because of their relatively low likelihood of worsening motor symptoms. Although clozapine is considered the drug of choice, it is underused in this population because of the need for frequent monitoring. Quetiapine, on the other hand, is generally first-line treatment despite its questionable efficacy. Consequently, in 2006, the American Academy of Neurology identified a need for the development of a novel antipsychotic with evidence of both safety and efficacy in patients with PDP. Pimavanserin, which has shown promise in clinical trials, recently became the first agent to receive FDA approval for the treatment of PDP.
Parkinson’s disease (PD), which was first described in 1817 by English surgeon and apothecary James Parkinson, is a chronic, slowly progressing neurodegenerative disease that affects as many as 1 million Americans.1,2 According to the Parkinson’s Disease Foundation, as many as 60,000 Americans are diagnosed with this often-debilitating disorder each year.2 Although it typically is not a direct cause of death, PD is associated with many complications and comorbidities that have led to increasing rates of mortality. The CDC reported that death rates for men and women with PD increased from 8.8 to 11 and from 3.9 to 4.8 per 100,000 people, respectively, from 2000 to 2013.3
Motor and Nonmotor Symptoms of PD
At its core, PD is characterized by four cardinal symptoms: bradykinesia, rigidity, resting tremor, and postural instability.4 Along with these typical motor symptoms come many nonmotor symptoms with significant associated morbidity and mortality. These include autonomic dysfunction, disorders of sleep and wakefulness, cognitive dysfunction and dementia, mood disorders, and psychosis.5 These nonmotor symptoms of PD are responsible for a significant proportion of hospitalizations, with psychosis reportedly accounting for 24% of hospital admissions in patients with PD.6 This fact signifies the importance of properly managing patients with PD psychosis (PDP) on both an inpatient and an outpatient basis.6
Parkinson’s Disease Psychosis
The most common symptom of PDP is visual hallucinations, which affect up to 40% of PD patients. Delusions, paranoia, panic attacks, and auditory hallucinations are less common.6 It is estimated that up to 60% of PD patients experience psychosis, with older patients and those requiring higher doses of dopaminergic drugs at higher risk.7 Diagnostic criteria for PDP have been established because of the complex differential diagnosis for psychosis. To be diagnosed with PDP, a patient must have all of the following: at least one psychotic symptom; a primary diagnosis of PD; symptoms that are recurrent or continuous for at least 1 month and occur after PD onset; and no other causes of psychosis. Additionally, associated features of PD, such as dementia, and PD drug treatment must be specified.8 It has been reported that up to 80% of PD patients develop dementia, which confounds the accurate diagnosis of PDP versus the behavioral issues associated with dementia.7
The etiology of PDP, which is not clear-cut, involves a potentially complex interaction between internal and external factors.9 It was previously thought that psychosis in PD patients was primarily iatrogenic—i.e., caused by the medications being used to treat the PD symptoms. On a basic level, psychosis and PD may be considered opposite ends of the dopamine spectrum. Whereas dopamine antagonism is a mechanism employed by medication in the treatment of psychosis, dopaminergic agents are used in the treatment of PD. Of the different subtypes of dopamine receptors, only the D2 receptor is seen in both the mesolimbic/mesocortical pathway, where it is associated with development of psychosis, and the nigrostriatal pathway, where the motor side effects of PD can originate. Therefore, this receptor subtype appears to play a balancing role and has implications for the management of PDP. Other signaling pathways have been linked to the pathophysiology of PDP and point to the disease process of PD as a contributor to the psychosis. These neurotransmitter systems, including serotonin and acetylcholine, are potential targets for drug therapy.9 A 2016 cross-sectional study found that 11% of drug-naïve PD patients had psychosis, supporting the role of the PD process in psychosis development independent of drug-therapy effects.10
Overview of PDP Management
Physical Versus Emotional Control: The intertwining pathophysiology of psychosis and PD through dopaminergic pathways presents healthcare professionals and patients with the unfortunate choice between physical and emotional stability. Dopaminergic agents that treat the symptoms of PD and maintain physical control are predominately associated with the triggering of psychosis symptoms through D2-receptor activation.9,11 This swing to emotional instability could be broadly treated in one of two ways. One option is to stop the anti-PD agent; however, this is not feasible for most patients because physical instability and motor symptoms would return. Alternatively, an antipsychotic could be added, but nearly all typical and atypical antipsychotics work via D2-receptor antagonism, potentially tipping the scale toward physical instability. Accordingly, methods used in practice involve dose reduction of offending agents, as tolerated, or the use of an atypical antipsychotic with low D2-receptor affinity.9,11
Complexities Surrounding Antipsychotic Use: The use of currently available antipsychotics in this population is complex, in part because of the black box warning regarding the increased risk of death when these agents are used in elderly patients with dementia.12 This is significant for patients with PD for a multitude of reasons. First, PD is highly associated with the development of dementia, so many patients who are treated for psychosis likely have comorbid dementia. Additionally, psychotic symptoms of PD are more prominent with age, indicating a higher need for therapy in older patients. Even without the codiagnosis of dementia, antipsychotic use has been associated with a higher incidence of death in the PD population (hazard ratio 2.35, P <.001).13 Although these medications have an increased risk of all-cause mortality, the exact mechanism has not been determined. Given the lack of alternative options, the risk of mortality must be balanced against the high morbidity and mortality associated with psychosis itself,14 and antipsychotics remain the standard of practice for PDP treatment.
Stepwise Approach: The initial step in the treatment of PDP is to rule out external or secondary causes, such as infection, metabolic abnormalities, or structural lesions of the brain.9,15 The next step, which is crucial for pharmacists, is to review the patient’s current medications and identify any agents that may exacerbate psychotic symptoms. This includes not only anti-PD agents, but also medications for comorbid conditions. Potentially offending agents that are deemed nonessential should be discontinued. These may include tricyclic antidepressants, bladder antispasmodics, anticholinergics, benzodiazepines, muscle relaxants, and opioids—all of which are associated with some level of psychosis.9,16
If psychotic symptoms persist, it is important to review the anti-PD agents being used. Guidelines have suggested the reduction or discontinuation of anti-PD medications, starting with those with the most potential for psychosis induction and the least anti-PD activity.16 Which agent to reduce or discontinue may be determined according to the following order: anticholinergics, amantadine, selegiline, dopamine agonists, and levodopa/carbidopa. Once the decision has been made to alter the use of a dopaminergic agent, it is vital to ensure that this medication is tapered gradually to avoid withdrawal and prevent neuroleptic malignant syndrome.16,17 When anti-PD agents are being reduced, levodopa doses may need to be increased accordingly to maintain motor control.17 If the above options do not resolve psychotic symptoms while maintaining motor control, the addition of an antipsychotic should be considered. TABLE 1 reviews the treatment algorithm for medical management of PDP. Above all, the patient and his or her caregiver should be closely involved in the treatment decision, fostering a patient-centered, individualized approach that emphasizes the risks and benefits specific to the patient.
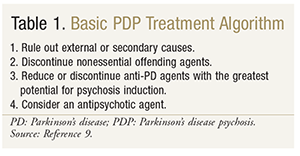
Antipsychotic Treatment
Atypical Antipsychotics: The typical antipsychotics are widely known to exacerbate motor symptoms in PD patients and thus play virtually no role in the treatment of PDP, except in acute circumstances when neuropsychiatric control is necessary over motor control.11,18 The underlying mechanism of motor-symptom exacerbation is likely related to high D2-receptor antagonism.11 Although D2-receptor affinity crosses over to the atypical antipsychotics as well, in clinical studies two atypical antipsychotics (clozapine and quetiapine) with low D2-receptor affinity have demonstrated improved psychotic symptoms without dramatically worsened motor symptoms.9,16 Other atypical antipsychotics are not considered for use in PDP because of their high likelihood of worsening motor symptoms. Other neurotransmitter systems linked to PDP, such as serotonin, are impacted by atypical antipsychotics and likely contribute to their efficacy in this population. See TABLE 2 for receptor-binding affinities of selected atypical antipsychotics.19
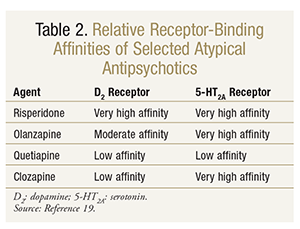
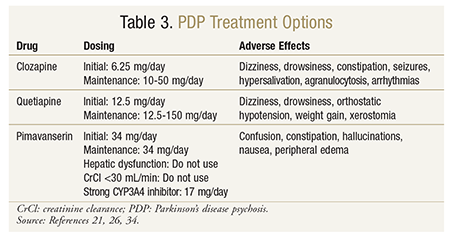
Clozapine: Clozapine was the first atypical antipsychotic approved for use in the United States.20 Clozapine is indicated for schizophrenia and schizoaffective disorder, but in clinical trials it was shown to consistently improve symptoms of PDP without worsening motor control.9,21-24 Clozapine is a dibenzothiazepine derivative classically described as a dopamine antagonist with a low affinity for D2 receptors and a higher affinity for D1, D3, D4, and 5-HT2A (serotonin) receptors.20 Additionally, clozapine has rapid dissociation from the D2 receptor.20 The combination of low D2-receptor affinity and high 5-HT2A receptor binding likely accounts for clozapine’s effectiveness and absence of motor-function aggravation. Clozapine is also associated with adrenergic, cholinergic, and histaminergic antagonism, which probably explains its related adverse effects. Dosing in the setting of PDP is significantly lower than that typically used in the treatment of schizophrenia, which may additionally explain clozapine’s lack of aggravation of motor symptoms. Dosages studied in clinical trials ranged from 6.25 mg to 50 mg, with mean dosages of 25 mg/day.9,20,22-24 See TABLE 3 for a summary of clozapine in the treatment of PDP.
In 2006, the American Academy of Neurology (AAN) released guidelines stating that clozapine should be considered for PD patients with psychosis, but that the absolute neutrophil count (ANC) must be monitored.14 The 2011 guidelines of the Movement Disorder Society (MDS) concluded that clozapine is efficacious, noting that it has an acceptable safety risk and requires specialized monitoring.5 Monitoring includes frequent WBC count testing to assess for rare but potentially life-threatening agranulocytosis, which occurs in 0.38% of patients.5 Because of this risk, clozapine has a Risk Evaluation and Mitigation Strategies program that recommends ANC monitoring weekly from drug initiation to 6 months, followed by biweekly monitoring from 6 to 12 months, and then monthly monitoring thereafter. More frequent monitoring is required if a patient develops neutropenia.25 Most physicians and pharmacists have insufficient experience with clozapine because of the need for hematologic monitoring, which is a burden for patients and practitioners and results in the underuse of clozapine in the clinical setting.
Quetiapine: This dibenzothiazepine derivative is indicated for schizophrenia, bipolar disorder, mania, and adjunctively for depression.26 Quetiapine is associated with a relatively higher affinity for 5-HT2A receptors than for D2 receptors, which makes it another option for the treatment of PDP.20 Its high affinity for adrenergic and histaminergic receptors explains common adverse events such as orthostatic hypotension and sedation.20 In a recent systematic review of randomized, controlled trials involving quetiapine for PDP, mean daily dosages of quetiapine ranged from 58.3 mg to 169.1 mg.27 The suggested initial dosage is 12.5 mg daily, and it may be titrated to a maintenance dose of up to 150 mg.9 See TABLE 3 for a summary of quetiapine in the treatment of PDP. More reports of worsening PD symptoms have been associated with quetiapine than with clozapine. In the systematic review mentioned above, 10 of 122 patients receiving quetiapine experienced PD deterioration, but the total dropout rate of 32.4% may have led to underestimation of this effect.27
Based on inconsistent and conflicting efficacy results in open-label and randomized, controlled trials with both placebo and clozapine as comparators,28 the MDS guidelines concluded that evidence was insufficient to recommend quetiapine for PDP.5 Similar to clozapine, quetiapine was considered to have an acceptable safety risk; unlike clozapine, however, specialized monitoring is not required.5 The AAN guidelines stated that quetiapine may be considered for PDP, based on a study demonstrating possible improvement of psychosis.14 Although clinical studies have yet to definitively demonstrate its benefit, quetiapine is generally regarded as first-line therapy because of safety and monitoring concerns associated with clozapine.29
Pimavanserin: The 2006 AAN guidelines identified the need for evidence of efficacy of novel antipsychotics without dopamine antagonistic effects.14 Pimavanserin acts as an inverse agonist on 5-HT2A receptors and has no significant effect on dopaminergic, muscarinic, histaminic, or adrenergic receptors.29,30 Clinical studies have demonstrated both safety and efficacy without worsening of motor function. A 28-day randomized, placebo-controlled, double-blind, phase II trial with a 4-week follow-up period found no statistically significant difference in motor function based on the Unified Parkinson’s Disease Rating Scale. Efficacy, as evaluated with the Scale for the Assessment of Positive Symptoms (SAPS), showed statistically significant improvement in the global rating of both hallucinations and delusions. Trends were seen in other areas of the SAPS. No significant differences with regard to adverse events were seen between the placebo and pimavanserin groups.29
A 6-week randomized, placebo-controlled, phase III trial of 199 patients had similar results, with no evidence of treatment-related impairment of motor function between the placebo and pimavanserin groups. The study reached its primary endpoint of antipsychotic benefit for pimavanserin compared with placebo, which was assessed by a PD-adapted SAPS (SAPS-PD). There was a comparative mean decrease of 3.06 on the SAPS-PD scale for pimavanserin versus placebo (P = .001) and an overall decrease of 37% from baseline.31 A validation study for the SAPS-PD scale found that a reduction of 2.33 is considered clinically significant based on a one-point improvement on the Clinical Global Impression–Improvement scale, a seven-point, clinician-driven assessment of a patient’s overall mental illness.32 These results demonstrate that pimavanserin may have significant benefit in clinical practice. Pimavanserin was generally well tolerated, although 10 pimavanserin-treated patients discontinued treatment because of an adverse event (four of which were due to psychosis) versus two discontinuations for adverse events in the placebo group.31 A meta-analysis of four randomized, controlled trials of pimavanserin—including the two mentioned above and two unpublished studies—concluded that pimavanserin is beneficial for the treatment of PDP and has good tolerability.9 Pimavanserin has not been studied in any head-to-head trials with clozapine or quetiapine.
Based on the promising results of the phase II and phase III trials, the FDA approved pimavanserin (Nuplazid) on April 29, 2016, as the first drug indicated to treat hallucinations and delusions associated with PDP.33 Nuplazid, which is available as 17-mg tablets, has a recommended dosage of 34 mg taken orally once daily, with no titration needed; it may be taken with or without food. As with other antipsychotics, pimavanserin carries a black box warning regarding increased mortality in elderly patients with dementia-related psychosis. No study has directly linked pimavanserin to this risk, but the FDA considers the risk a class effect. Pimavanserin can cause QT interval prolongation and is metabolized by the CYP3A4 enzyme, so caution should be exercised. If a strong CYP3A4 inhibitor is used, the dosage should be reduced to 17 mg per day. Pimavanserin is not recommended in patients with hepatic impairment or severe renal impairment (creatinine clearance <30 mL/min).34 See TABLE 3 for a summary of pimavanserin in the treatment of PDP.
The high cost of pimavanserin will likely limit prescribing, and as with many name-brand products currently on the market, the manufacturer is currently offering a program to assist patients with the insurance-approval process.35,36 Because of the safety, efficacy, and lack of motor worsening seen in clinical trials, pimavanserin shows promise; however, with no head-to-head trial data available, it is difficult to ascertain this agent’s exact fit in current therapy.
Conclusion
PDP is a significant nonmotor manifestation of PD that presents primarily as hallucinations and delusions. Because of its complex etiology involving endogenous and exogenous factors and neurotransmitter pathways associated with the pathophysiology of both PD and psychosis, treatment options are limited. In many cases, a patient’s physical and emotional stability must be balanced. The treatment of PDP should follow a stepwise approach, and the patient and his or her caregiver should be closely involved in the treatment decision. When antipsychotic therapy is indicated, to minimize the worsening of motor symptoms, emphasis should be placed on using an agent with low D2-receptor affinity. Atypical antipsychotics with this property include quetiapine and clozapine. Pimavanserin, a novel agent with proven efficacy that does not worsen motor symptoms in PDP patients, is the first drug approved for the treatment of PDP and may be an appropriate option for patients who can afford it.
REFERENCES
1. Lai BC, Tsui JK. Epidemiology of Parkinson’s disease. BCMJ. 2001;43:133-137.
2. Parkinson’s Disease Foundation. Statistics on Parkinson’s. www.pdf.org/en/parkinson_statistics. Accessed October 4, 2016.
3. CDC. QuickStats: age-adjusted death rates for Parkinson disease—United States, 2000-2013. www.cdc.gov/mmwr/preview/mmwrhtml/mm6436a9.htm. Accessed October 4, 2016.
4. Gelb DJ, Oliver E, Gilman S. Diagnostic criteria for Parkinson disease. Arch Neurol. 1999;56:33-39.
5. Seppi K, Weintraub D, Coelho M, et al. The Movement Disorder Society evidence-based medicine review update: treatments for the non-motor symptoms of Parkinson’s disease. Mov Disord. 2011;26(suppl 3):S42-S80.
6. Aminoff MJ, Christine CW, Friedman JH, et al. Management of the hospitalized patient with Parkinson’s disease: current state of the field and need for guidelines. Parkinsonism Relat Disord. 2011;17:139-145.
7. Forsaa EB, Larsen JP, Wentzel-Larsen T, et al. A 12-year population-based study of psychosis in Parkinson disease. Arch Neurol. 2010;67:996-1001.
8. Ravina B, Marder K, Fernandez HH, et al. Diagnostic criteria for psychosis in Parkinson’s disease: report of an NINDS, NIMH work group. Mov Disord. 2007;22:1061-1068.
9. Chang A, Fox SH. Psychosis in Parkinson’s disease: epidemiology, pathophysiology, and management. Drugs. 2016;76:1093-1118.
10. Isais-Millán S, Piña-Fuentes D, Guzmán-Astorga C, et al. Prevalence of neuropsychiatric disorders in drug-naive subjects with Parkinson’s disease (PD) [in Spanish]. Gac Med Mex. 2016;152:357-363.
11. Shin HW, Chung SJ. Drug-induced parkinsonism. J Clin Neurol. 2012;8:15-21.
12. FDA. Public health advisory: deaths with antipsychotics in elderly patients with behavioral disturbances. www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformation forPatientsandProviders/ucm053171.htm. Accessed October 4, 2016.
13. Weintraub D, Chiang C, Kim HM, et al. Association of antipsychotic use with mortality risk in patients with Parkinson disease. JAMA Neurol. 2016;73:535-541.
14. Miyasaki JM, Shannon K, Voon V, et al. Practice parameter: evaluation and treatment of depression, psychosis, and dementia in Parkinson disease (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2006;66:996-1002.
15. Goldman JG, Vaughan CL, Goetz CG. An update expert opinion on management and research strategies in Parkinson’s disease psychosis. Expert Opin Pharmacother. 2011;12:2009-2024.
16. Olanow CW, Watts RL, Koller WC. An algorithm (decision tree) for the management of Parkinson’s disease (2001): treatment guidelines. Neurology. 2001;56(11 suppl 5):S1-S88.
17. Keyser DL, Rodnitzky RL. Neuroleptic malignant syndrome in Parkinson’s disease after withdrawal or alteration of dopaminergic therapy. Arch Intern Med. 1991;151:794-796.
18. Rochon PA, Stukel TA, Sykora K, et al. Atypical antipsychotics and parkinsonism. Arch Intern Med. 2005;165:1882-1888.
19. Casey DE, Zorn SH. The pharmacology of weight gain with antipsychotics. J Clin Psychiatry. 2001;62(suppl 7):4-10.
20. Farah A. Atypicality of atypical antipsychotics. Prim Care Companion J Clin Psychiatry. 2005;7:268-274.
21. Clozapine. In: Clinical Pharmacology. Tampa, FL: Gold Standard; 2016. www.clinicalpharmacology.com. Accessed October 4, 2016.
22. The Parkinson Study Group. Low-dose clozapine for the treatment of drug-induced psychosis in Parkinson’s disease. N Engl J Med. 1999;340:757-763.
23. Pollak P, Tison F, Rascol O, et al. Clozapine in drug induced psychosis in Parkinson’s disease: a randomised, placebo controlled study with open follow up. J Neurol Neurosurg Psychiatry. 2004;75:689-695.
24. Factor SA, Friedman JH, Lannon MC, et al. Clozapine for the treatment of drug-induced psychosis in Parkinson’s disease: results of the 12 week open label extension in the PSYCLOPS trial. Mov Disord. 2001;16:135-139.
25. Clozapine and the risk of neutropenia: a guide for healthcare providers. www.clozapinerems.com/CpmgClozapineUI/rems/pdf/resources/ANC_Table.pdf. Accessed October 4, 2016.
26. Quetiapine. In: Clinical Pharmacology. Tampa, FL: Gold Standard; 2016. www.clinicalpharmacology.com. Accessed October 4, 2016.
27. Desmarais P, Massoud F, Filion J, Nguyen QD, Bajsarowicz P. Quetiapine for psychosis in Parkinson disease and neurodegenerative parkinsonian disorders: a systematic review. J Geriatr Psychiatry Neurol. 2016;29(4):227-236.
28. Frieling H, Hillemacher T, Ziegenbein M, et al. Treating dopamimetic psychosis in Parkinson’s disease: structured review and meta-analysis. Eur Neuropsychopharmacology. 2007;17:165-171.
29. Meltzer HY, Mills R, Revell S, et al. Pimavanserin, a serotonin(2A) receptor inverse agonist, for the treatment of Parkinson’s disease psychosis. Neuropsychopharmacology. 2010;35:881-892.
30. Pimavanserin. In: Clinical Pharmacology. Tampa, FL: Gold Standard; 2016. www.clinicalpharmacology.com. Accessed October 4, 2016.
31. Cummings J, Isaacson S, Mills R, et al. Pimavanserin for patients with Parkinson’s disease psychosis: a randomised, placebo-controlled phase 3 trial. Lancet. 2014;383:533-540.
32. Voss T, Bahr D, Cummings J, et al. Performance of a shortened Scale for Assessment of Positive Symptoms for Parkinson’s disease psychosis. Parkinsonism Relat Disord. 2013;19(3):295-299.
33. FDA. FDA approves first drug to treat hallucinations and delusions associated with Parkinson’s disease. Accessed October 4, 2016.
34. Nuplazid (pimavanserin) package insert. San Diego, CA: Acadia Pharmaceuticals Inc; April 2016.
35. Pimavanserin. In: Lexicomp Online. Hudson, OH: Lexi-Comp, Inc; 2016. http://online.lexi.com. Accessed October 4, 2016.
36. Nuplazid. Acadia Pharmaceuticals Inc. www.nuplazid.com/connect/overview/. Accessed July 30, 2016.
To comment on this article, contact rdavidson@uspharmacist.com.






