US Pharm. 2019;44(9):32-35.
ABSTRACT: Postmenopausal osteoporosis affects millions of women each year. Initial treatment is based on many factors including T-score, fracture risk assessment tool score, and risk factors for fractures. The most recent additions to treatment are aimed at those who are considered to be at higher fracture risk. The newest FDA-approved medications, abaloparatide and romosozumab, work to increase bone formation in 18 and 12 months, respectively. Abaloparatide has shown a statistically significant effect in preventing vertebral fractures compared with placebo. Studies using romosozumab have shown significantly increased bone mineral density compared with teriparatide.
Osteoporosis is characterized by a loss of bone density and mass causing an increased risk of fractures.1 Approximately 10 million people in the United States have been diagnosed with this condition; 80% are women.2 The decrease in estrogen levels after the start of menopause causes a sustained drop in bone density in most women. The most common osteoporosis-related fractures occur in the hip, spine, and wrists. These types of fractures can be extremely debilitating and lead to many complications. The consequences of osteoporosis are significant in terms of its overall cost to the healthcare system. The National Osteoporosis Foundation estimates $19 billion in direct and indirect costs related to fractures yearly.1 New treatment agents recently approved by the FDA, abaloparatide and romosozumab, offer additional therapy options for patients with a high fracture risk or for whom previous therapies have failed.
DIAGNOSTIC CRITERIA
Diagnosis of osteoporosis may include a low T-score measured by a dual x-ray absorptiometry scan. A T-score compares the patient’s bone mineral density (BMD) to the average BMD in a healthy, young adult. A lumbar spinal, 33% radius of forearm, femoral neck, or hip T-score that is <-2.5 is considered diagnostic. Fragility fractures are another diagnostic criterion for osteoporosis, defined by a fracture that occurs from minimal trauma or simple activities of daily life. Finally, a fracture risk assessment tool (FRAX) score may be used to confirm the diagnosis and need for pharmacotherapy. The FRAX algorithm calculates a 10-year probability of a major osteoporotic fracture for each patient.3 The American Association of Clinical Endocrinology and American College of Endocrinology (AACE/ACE) 2016 Guidelines for the Treatment of Postmenopausal Osteoporosis recommend pharmacotherapy for those with osteopenia (T-score between -1.0 and -2.5) and a history of fragility fracture; osteopenia with a FRAX 10-year probability >3% for hip fracture or >20% for other major osteoporotic fracture; or T-score at any location <-2.5. Osteoporosis may also be diagnosed with a history of a low-trauma fracture, regardless of T-score.3
OVERVIEW OF CURRENT THERAPY
Nonpharmacologic Recommendations
AACE/ACE guidelines recommend lifestyle modifcations and nonpharmacologic therapies for all patients diagnosed with osteoporosis. Laboratory values for calcium and vitamin D should be evaluated prior to initiating pharmacotherapy.3 Goal serum 25-hydroxy vitamin D levels should be maintained at 30-50 ng/mL, and patients older than age 50 years should have a target intake of 1,000-2,000 IU of vitamin D3 daily. Calcium intake should be evaluated and maintained at 1,200 mg per day for women older than age 50 years. Overall, dietary intake of vitamin D3 and calcium is preferred over supplements; however, if this is not feasible, supplement use should be recommended.
Other lifestyle recommendations include participation in weight-bearing, balance, and resistance exercise as appropriate. Smoking and using other tobacco products should be avoided, and alcohol intake of less than two standard drinks daily is recommended. All patients should be assessed for fall risks, including a thorough evaluation of medication therapies that may put the patient at higher risk for a fall.3 Benzodiazepines, antihypertensives, narcotics, and atypical antipsychotics are associated with dizziness and hypotension and, therefore, should be used with caution in this population.
Current Medication Therapies
The 2019 Journal of Clinical Endocrinology and Metabolism (JCEM) Clinical Practice Guidelines for pharmacological ma
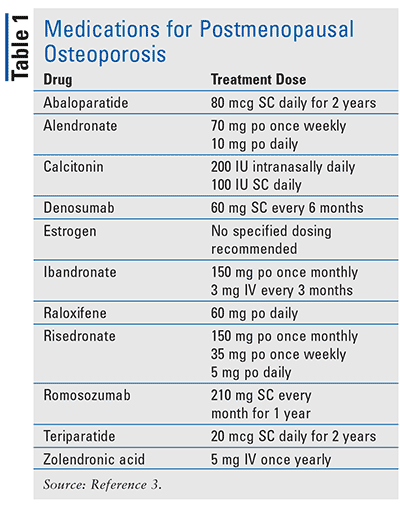
nagement of osteoporosis in postmenopausal women state that oral bisphosphonates such as alendronate and risedronate should be considered as first-line therapy for those at high fracture risk (Table 1 lists medications and dosages for postmenopausal osteoporosis).4 JCEM guidelines recommend denosumab, teriparatide, or abaloparatide for patients at high to very high fracture risk. High risk is described as a prior spine or hip fracture; a T-score at hip or spine of <-2.5 or below (the more negative the number, the greater the risk); 10-year hip fracture risk >3%; or risk of major osteoporotic fracture >20%. Patients are considered at very high risk if a history of multiple spinal fractures is present, along with a diagnostic T-score. Other reasons injectable medications can be recommended over oral bisphosphonates include nonadherence to oral therapy, esophageal disease that could be exacerbated by oral bisphosphonates, or gastrointestinal problems that would prevent absorption.3,4 Denosumab is the agent of choice for patients with renal insufficiency, because it is not renally excreted.3
AACE/ACE guidelines identify alendronate, risedronate, zoledronic acid, and denosumab as initial choices for therapy for those with no history of low-impact fractures or a moderate fracture risk.3 Alternate treatment options could include ibandronate or raloxifene. Patients with a history of prior fragility fractures or higher fracture risk should consider denosumab, teriparatide, or zoledronic acid as first-line treatment options.3 Higher fracture risk is identified by a combination of advanced age, frailty, history of long-term glucocorticoid use, very low T-scores, or increased fall risk. Other risk factors that can be considered when assessing fracture risk are premature menopause, primary or secondary amenorrhea, Asian or Caucasian ethnicity, family or personal history of a low-impact fracture, low body weight, smoking history, excess alcohol use, long-term immobilization, and poor diet.5 If the patient has a significant decrease in BMD or a new fragility fracture despite current treatment, this is considered treatment failure, and therapy with an alternate drug class is warranted.3
Though less commonly used due to reduced efficacy or increased risk of adverse effects, raloxifene, calcitonin, and estrogen may be used to treat postmenopausal osteoporosis.3,4 Raloxifene can be used if the patient cannot use bisphosphonates or denosumab, is at low risk of venous thromboembolism, and has a high risk of breast cancer.4 Nasal-spray calcitonin may be beneficial to reduce the risk of vertebral fractures only. Estrogen therapy may be used to prevent postmenopausal osteoporosis and reduce fracture risk. Due to the risk of thromboembolism, estrogen should be used in the lowest effective dose for the shortest amount of time possible.3
New Medication Therapies
Abaloparatide: The FDA approved abaloparatide for treatment in postmenopausal women at high fracture risk in April 2017. This adds another parathyroid hormone analogue to the market in addition to teriparatide.6,7 Abaloparatide binds with a much higher affinity to the transient RG conformation of the parathyroid hormone type 1 receptor, compared with teriparatide. This mechanism may increase bone formation and decrease bone resorption more than teriparatide.8 The dosing for abaloparatide is 80 mcg injected subcutaneously daily.7 Reported adverse effects include dizziness, hypercalcemia, hyperuricemia, and injection-site reactions, which occurred in more than 10% of the population receiving abaloparatide.7 It is recommended that abaloparatide be administered in a setting where the patient can sit or lie down if an episode of hypotension occurs.
Miller and colleagues conducted a randomized, clinical trial comparing the efficacy of abaloparatide versus placebo or open-label teriparatide in preventing vertebral fractures.9 The primary endpoint was percentage of patients with one or more new morphometric fractures. The difference in new morphometric vertebral fractures in the abaloparatide group was significantly lower than that of the placebo group at 18 months (P <.001).9 A statistical comparison between abaloparatide and teri-paratide on the primary outcome was not done due to the inability to meet power for this comparison. However, abaloparatide showed a greater increase in BMD by month 6 of treatment compared with teriparatide (P <.001).9 Incidence of hypercalcemia was significantly lower in the group receiving abaloparatide compared with the group receiving teriparatide at all time points (P = .006), a result Miller and colleagues associated with less bone resorption.9 Currently, abaloparatide is available for a significantly lower average wholesale price than teriparatide. Additionally, efficacy in fracture reduction with abaloparatide at 18 months was similar to efficacy of teriparatide at 24 months of therapy; this shorter length of therapy overall will result in cost savings to the patient.
Abaloparatide is available as a 2,000 mcg/1 mL solution pen containing 1.56 mL for a total of thirty 80-mcg doses.7 Pens should be stored at a refrigerated temperature long-term but can also be stored at temperatures up to 77°F if used in 1 month. This differs from teriparatide, which must be refrigerated at all times.7,10 Patients should be trained on proper injection technique as abaloparatide is administered at home. Injections should occur in the periumbilical region of the abdomen and injection sites rotated daily.6 Owing to the possibility of hypercalcemia and urolithiasis, serum calcium and uric acid should be monitored during therapy.7 The FDA requires a boxed warning on abaloparatide for an increased risk of osteosarcoma that occurred in rats. The doses given to rats were 4 to 28 times the human exposure that occurs with the 80-mcg daily dose. Although this effect has not been seen in humans, use of abaloparatide should be limited to 2 years in all patients and is not recommended in patients with Paget’s disease or who are at increased risk of osteosarcoma.6 Following 2 years of therapy with either teriparatide or abaloparatide, it is recommended to use antiresorptive agents such as bisphosphonates or denosumab to preserve BMD gains.3,4
Romosozumab: In April 2019, the FDA approved romosozumab for osteoporosis in postmenopausal women at high fracture risk.11 Prescribing information for romosozumab defines high fracture risk as a history of osteoporotic fracture, multiple risk factors for fracture, and having failed or been intolerant to other therapies.12 The mechanism of action for romosozumab is unlike any currently on the market. Romosozumab is a humanized monoclonal antibody and sclerostin inhibitor, promoting the Wnt pathway that increases bone formation and decreases bone resorption. Sclerostin is responsible for inhibiting the Wnt pathway and decreasing overall bone formation.13 Dosing is 210 mg via SC injection once monthly for 12 months. Use is limited to 12 months as studies evaluating bone turnover markers demonstrated a waning effect after this treatment period.12 Romosozumab is supplied as a 105 mg/1.17 mL prefilled syringe solution for injection. Two syringes should be administered consecutively in-office by a healthcare provider monthly.11 This product must be kept refrigerated when not in use and must be disposed of if left at room temperature for 30 days or longer.
A phase III, randomized, controlled trial enrolled 7,180 postmenopausal women to receive either romosozumab or placebo for 12 months. Each treatment arm then received 12 months of open-label treatment with denosumab. This trial resulted in a statistically significant reduction in new vertebral fractures and clinical fractures in patients treated with romosozumab compared with placebo at 12 months. At the 24-month mark, those treated with romosozumab had a 75% lower risk of vertebral fractures compared with placebo.14
Another phase III, randomized, controlled trial conducted by Saag and colleagues studied the effectiveness of romosozumab versus alendronate for 12 months, followed by open-label treatment with alendronate for both groups for an additional 12 months. This study found that treatment with romosozumab transitioned to alendronate to be superior to treatment with alendronate alone, yielding a 48% lower risk in new vertebral fractures, 27% lower risk of clinical fractures, and a 38% lower risk of hip fractures, all statistically significant compared with the control group (P <.001, P <.001, P = .02, respectively).15
Efficacy of romosozumab versus teriparatide has been analyzed in a phase III, open-label, randomized, controlled trial conducted by Langdahl and colleagues. A total of 415 patients who received oral bisphosphates in the past 3 years prior to the trial were randomized to either romosozumab 210 mg SC monthly or teriparatide 20 mcg SC daily for 12 months. The primary outcome of mean percent change in baseline BMD at the total hip up to month 12 of treatment showed a significant increase in the group receiving romosozumab (+2.6%) versus the teriparatide group (-0.6%; P <.0001). Analysis of secondary outcomes at months 6 and 12 also showed significantly greater increases in mean percent change in BMD at femoral neck and lumbar spine compared with teriparatide. Data were not gathered on fracture incidence in these two groups.16
Side effects occurring in more than 10% of the population receiving romosozumab include antibody formation and arthralgia.11 In the trial conducted by Cosman and colleagues, arthralgias occurred in 15.8% of the patients receiving placebo and 16.3% of patients receiving romosozumab.14 As with any biologic agent, antibody production with the potential to neutralize the drug is possible. Both aforementioned studies reported a small percentage of patients tested positive for neutralizing antibodies (0.7% and 0.6%, respectively).14,15
The FDA requires a boxed warning for this medication owing to a higher rate of major adverse cardiac events. In the study conducted by Saag and colleagues, 2.5% of patients receiving romosozumab and 1.9% of patients receiving alendronate reported serious cardiovascular events during the double-blind period (95% CI, 0.85-2.00).15 Therefore, romosozumab should not be administered to those who have had a myocardial infarction or stroke in the previous year, and it should be used with caution in those who have cardiac risk factors. Romosozumab is contraindicated in hypocalcemia and in those with a known hypersensitivity to romosozumab or any ingredient present in the solution. Serum calcium must be monitored during treatment with romosozumab.12
CONCLUSION
Postmenopausal osteoporosis is a chronic condition affecting millions of women worldwide. Use of newer agents for osteoporosis treatment such as abaloparatide and romosozumab offer alternatives for those for whom traditional therapy has not been successful or who are at a high fracture risk. A patient’s individual risk factors for fracture and history of prior treatment failure, the high cost of these new medications, and potential serious adverse reactions are all factors that are imperative to consider when selecting an appropriate osteoporosis treatment.
REFERENCES
1. National Osteoporosis Foundation. Learn what osteoporosis is and what causes it. www.nof.org/patients/what-is-osteoporosis/. Accessed August 14, 2019.
2. National Osteoporosis Foundation. What women need to know. www.nof.org/preventing-fractures/general-facts/what-women-need-to-know/. Accessed August 14, 2019.
3. Camacho PM, Petak SM, Binkley N, et al. AACE/ACE clinical practice guidelines. American Association of Clinical Endocrinologists. Published November 13, 2018. www.aace.com/publications/guidelines. Accessed August 14, 2019.
4. Eastell R, Rosen CJ, Black DM, et al. Pharmacological management of osteoporosis in postmenopausal women: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2019;104:1595-1622.
5. Kanis JA. Diagnosis of osteoporosis and assessment of fracture risk. Lancet. 2002;359(9321):1929-1936.
6. Tymlos (abalopararide) prescribing information. Waltham MA: Radius Health Inc; 2017.
7. Abaloparatide. Clinical pharmacology. [Internet database]. Tampa, FL: Gold Standard. 2019. www.clinicalpharmacology.com/. Accessed August 14, 2019.
8. Cosman F. The evolving role of anabolic therapy in the treatment of osteoporosis. Curr Opin Rheumatol. 2019;31(4):376-380.
9. Miller PD, Hattersley G, Riis BJ, et al. Effect of abaloparatide vs placebo on new vertebral fractures in postmenopausal women with osteoporosis. JAMA. 2016;316(7):722-733.
10. Forteo (teriparatide) prescribing information. Indianapolis, IN: Eli Lilly and Co; 2002.
11. Romosozumab. Clinical pharmacology [Internet database]. Tampa, FL: Gold Standard. 2019. www.clinicalpharmacology.com/.
12. Evenity (romosozumab aqqg) prescribing information. Thousand Oaks, CA: Amgen, Inc; 2019.
13. Lim SY, Bolster MB. Profile of romosozumab and its potential in the management of osteoporosis. Drug Des Devel Ther. 2017;11:1221-1231.
14. Cosman F, Crittenden DB, Adachi JD, et al. Romosozumab treatment in postmenopausal women with osteoporosis. N Engl J Med. 2016;375(16):1532-1543.
15. Saag KG, Petersen J, Brandi ML, et al. Romosozumab or alendronate for fracture prevention in women with osteoporosis. N Engl J Med. 2017;377(15):1417-1427.
16. Langdahl BL, Libanati C, Crittenden DB, et al. Romosozumab (sclerostin monoclonal antibody) versus teriparatide in postmenopausal women with osteoporosis transitioning from oral bisphosphonate therapy: a randomised, open-label, phase 3 trial. Lancet. 2017;390:1585-1594.
To comment on this article, contact rdavidson@uspharmacist.com.