Richard Mullvain, R.Ph., BCCP, BCPS (AQC), graduated from the University of Minnesota College of Pharmacy and has been a pharmacist with Essentia Health in Duluth, Minnesota, for almost 30 years. For the last 14 years he has been the STEMI program manager for one of the largest STEMI systems of care in the Upper Midwest. He currently has 18 publications related to heart attack care, and has a real passion to educate, reduce gender disparities in care, and improve the outcomes of patients suffering a heart attack.
Richard Mullvain, R.Ph., BCCP, BCPS (AQC), graduated from the University of Minnesota College of Pharmacy and has been a pharmacist with Essentia Health in Duluth, Minnesota, for almost 30 years. For the last 14 years he has been the STEMI program manager for one of the largest STEMI systems of care in the Upper Midwest. He currently has 18 publications related to heart attack care, and has a real passion to educate, reduce gender disparities in care, and improve the outcomes of patients suffering a heart attack.
How did a pharmacist become a STEMI Heart Attack Program Manager?
It started with our interventional cardiologists requesting standardization of medications used for heart attack care. Our large rural area had about 25 hospitals transferring patients with STEMI, and many of them used different doses and concentrations of IV unfractionated heparin, and had confusion over the dosing of enoxaparin, aspirin, and oral P2Y12 inhibitors. I was tasked to create a standardized regional STEMI protocol. This took a lot of travel, communication, and education with regional hospitals and ambulance services. As a pharmacist I was able to comfortably cite literature, data, and explain the rationale and evidence behind the drugs used in the protocol.
What do the latest national guidelines say about using IV KENGREAL® (cangrelor)?
The recently released 2021 ACC/AHA/SCAI Guideline for Coronary Artery Revascularization has a specific recommendation: In patients undergoing PCI who are P2Y12 inhibitor naïve, intravenous cangrelor may be reasonable to reduce periprocedural ischemic events (Class of Recommendation 2b with level of evidence B from randomized clinical trials).1 This update adds to our comfort level in using KENGREAL in the cath lab.
What is your “ten-thousand-foot” view of where KENGREAL fits into the cath lab?
KENGREAL is a very important tool in our toolbox to treat patients undergoing percutaneous coronary intervention (PCI).2 Cath labs performing primary PCI for STEMI patients should have quick access to KENGREAL for when it is needed.1,2
What clinical evidence supports your views to recommend KENGREAL?
I was really impressed with the CHAMPION PHOENIX outcomes data. CHAMPION PHOENIX was a randomized, double-blind, placebo- controlled, phase III trial in 11,145 patients who were undergoing either urgent or elective PCI and were receiving guideline-recommended therapy.3 Patients received a bolus and infusion of cangrelor or a loading dose of 600 mg or 300 mg of clopidogrel.3 It showed a 22% relative risk reduction in the primary endpoint of death, myocardial infarction, ischemia-driven revascularization, and stent thrombosis at 48 hours vs. clopidogrel (4.7% vs. 5.9%, P=0.005).3 I was even more impressed by the secondary endpoint, which showed a relative risk reduction of stent thrombosis by 38% at 48 hours vs. clopidogrel (0.8% vs 1.4%, P=0.01).3
How fast does KENGREAL inhibit platelets?
It’s quite impressive how the platelets are essentially “shut down” within just a few minutes of administering an IV bolus of KENGREAL.2 Adequate platelet inhibition is paramount to reduce the risk of events in the PCI procedure, including death, myocardial infarction, ischemia-driven revascularization, and stent thrombosis.3,4 With KENGREAL, platelet inhibition is sustained for the duration of the infusion.2 Then, equally important is the predictable recovery of platelets within only 1 hour after stopping the infusion, should the patient have bleeding complications or require urgent surgery.2
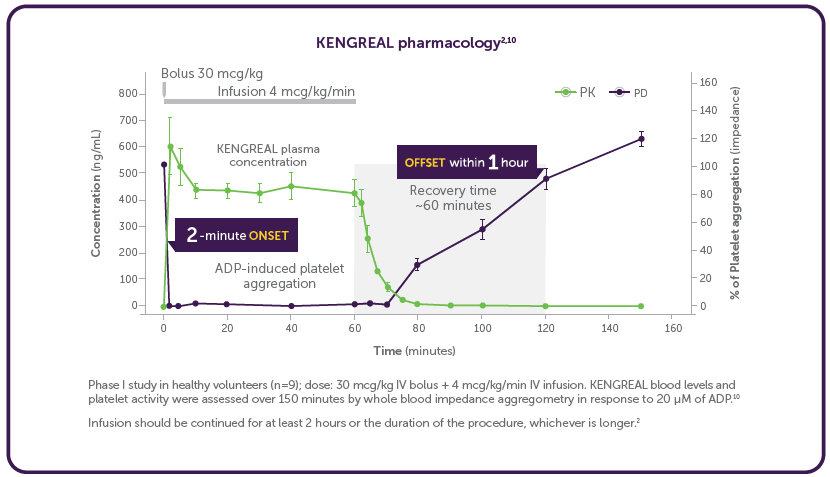
Phase I study in healthy volunteers (n=9); dose: 30 mcg/kg IV bolus + 4 mcg/kg/min IV infusion. KENGREAL blood levels and platelet activity were assessed over 150 minutes by whole blood impedance aggregometry in response to 20 μM of ADP.10
Infusion should be continued for at least 2 hours or the duration of the procedure, whichever is longer.2
Important Safety Information
Drugs that inhibit platelet P2Y12 function, including KENGREAL®, increase the risk of bleeding. In CHAMPION PHOENIX, bleeding events of all severities were more common with KENGREAL® than with clopidogrel.
Please see below for Full Important Safety Information.
What are common STEMI scenarios when you would say that KENGREAL should be considered for use in the cath lab?
STEMI cases where oral P2Y12 inhibitors have not been given prior to PCI is one scenario to consider using KENGREAL.5 Some patients are unable to take oral P2Y12 inhibitors for a variety of reasons, including intubation. Others vomit after taking oral aspirin and oral P2Y12 inhibitors. Another concern is patients who have been given opiates like morphine or fentanyl, which are often given to STEMI patients for pain management, and are also given routinely as a component of conscious sedation in the cath lab. This practice is well known to impair the absorption of oral medications; in fact, the oral P2Y12 inhibitors now all carry a warning in their FDA-approved prescribing information that states6-8:
Co-administration of opioid agonists delay and reduce the absorption…Consider the use of a parenteral anti-platelet agent in acute coronary syndrome patients requiring co-administration of morphine or other opioid agonists.
What are other scenarios where you have seen KENGREAL used in the cath lab?
I’ve seen cases where an acute coronary syndrome patient may already be known to us, or the cardiologist suspects multi-vessel coronary disease going into PCI, so they chose not to preload the patient with an oral P2Y12 inhibitor. Once the coronary anatomy is analyzed with angiography, a consult with a cardiothoracic surgeon is made to determine if the patient needs urgent or emergent coronary artery bypass graft (CABG) surgery. They then could choose to use KENGREAL if the patient proceeded to PCI. If CABG is needed and an oral P2Y12 is on board, the surgeon may require a “washout” period of up to 7 days to allow offset of the oral P2Y12 and avoid significant risk of bleeding.6-8
Finally, I have seen it used in patients with high-risk coronary anatomy features such as long, calcified, thrombotic lesions or multivessel PCI, which can increase the risk of major adverse cardiac events, as well as stent thrombosis.9 In a post hoc analysis of CHAMPION PHOENIX, the absolute benefit of KENGREAL increased progressively with the number of high-risk lesion features treated and was greatest for patients with three or more high-risk features.‡9 That analysis showed that about 25% of patients had three or more high-risk features.9
While KENGREAL is certainly not appropriate for every case, there are definitely circumstances and situations where it makes perfect sense.
Indication
KENGREAL® (cangrelor) for Injection is a P2Y12 platelet inhibitor indicated as an adjunct to percutaneous coronary intervention (PCI) to reduce the risk of periprocedural myocardial infarction (MI), repeat coronary revascularization, and stent thrombosis (ST) in patients who have not been treated with a P2Y12 platelet inhibitor and are not being given a glycoprotein IIb/IIIa inhibitor.
Important Safety Information
Drugs that inhibit platelet P2Y12 function, including KENGREAL®, increase the risk of bleeding. In CHAMPION PHOENIX, bleeding events of all severities were more common with KENGREAL® than with clopidogrel. Bleeding complications with KENGREAL® were consistent across a variety of clinically important subgroups. Once KENGREAL® is discontinued, there is no antiplatelet effect after an hour.
KENGREAL® (cangrelor) for Injection is contraindicated in patients with significant active bleeding.
KENGREAL® is contraindicated in patients with known hypersensitivity (e.g., anaphylaxis) to cangrelor or any component of the product.
The most common adverse reaction is bleeding.
Please see Full Prescribing Information
References: 1. Lawton JS, Tamis-Holland JE, Bangalore S, et al. 2021 ACC/AHA/SCAI Guideline for Coronary Artery Revascularization: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2021;00:000-000. 2. KENGREAL® (cangrelor) Prescribing Information. 2020. 3. Bhatt DL, Stone GW, Mahaffey KW, et al; CHAMPION PHOENIX Investigators. Effect of platelet inhibition with cangrelor during PCI on ischemic events. N Engl J Med. 2013;368(14):1303-1313. 4. Thel MC, Califf RM, Tardiff BE, et al. Timing of and risk factors for myocardial ischemic events after percutaneous coronary intervention (IMPACT-II). Am J Cardiol. 2000;85(2):427-434. 5. Ibanez B, James S, Agewall S, et al. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J. 2018;39:119-177. 6. EFFIENT® (prasugrel) Prescribing Information. 2019. 7. PLAVIX® (clopidogrel bisulfate) Prescribing Information. 2021. 8.BRILINTA® (ticagrelor) Prescribing Information. 2021. 9. Stone GW, Généreux P, Harrington RA, et al. Impact of lesion complexity on peri-procedural adverse events and the benefit of potent intravenous platelet adenosine diphosphate receptor inhibition after percutaneous coronary intervention: core laboratory analysis from 10,854 patients from the CHAMPION PHOENIX trial. Eur Heart J. 2018;39(46):4112-4121. 10. Akers WS, Oh JJ, Oestreich JH, Ferraris S, Wethington M, Steinhubl SR. Pharmacokinetics and pharmacodynamics of a bolus and infusion of cangrelor: a direct, parenteral P2Y12 receptor antagonist. J Clin Pharmacol. 2010;50(1):27-35.
KENGREAL® is a registered trademark of Chiesi Farmaceutici S.p.A.
©2022 Chiesi USA, Inc. All rights reserved. PP-K-0811 V1.0