US Pharm. 2010;35(5):Epub.
Chronic kidney disease (CKD) remains a growing problem in the United States. In 2000, an estimated 400,000 U.S. patients received either a kidney transplant or dialysis.1 This number is expected to rise to 2 million by 2030. Osteoporosis is another concern, with the incidence in individuals aged over 50 years ranging from 6% in men to 7.2% in women.2,3
Bisphosphonates, which are excreted via the kidneys, may accumulate in patients with diminished renal function, and toxicity may occur. There have been several reports of acute renal toxicity following bisphosphonate treatment. Seven bisphosphonates are approved for use in the U.S., each with its own characteristics and safety profile. There are few large, well-controlled studies to guide selection of the right bisphosphonate for a CKD or renal-transplant patient. Existing data regarding each agent’s effect on renal function, as well as use in patients with pre-existing renal impairment, are presented in the following review.
Zoledronate
Zoledronate is indicated for the treatment and prevention of osteoporosis in adults; the treatment and prevention of glucocorticoid-induced osteoporosis in patients expected to take glucocorticoids for at least 12 months; the treatment of Paget’s disease; and the treatment of hypercalcemia of malignancy and multiple myeloma in patients with documented bone metastases from solid tumors.4,5 Two long-term trials in cancer patients demonstrated an increase in serum creatinine (SCr) when zoledronate 8 mg was administered, prompting a recommendation to use 4 mg.6,7
Despite this measure, renal toxicity remains a possibility following treatment with zoledronate. Seventy-two cases of zoledronate-associated renal failure were reported to the FDA between August 2001 and March 2003; 27 patients required dialysis and 18 patients died.8 Other potential contributing factors to renal failure were not considered, however. A 2004 analysis of the French Adverse Event Reporting System found seven cases of acute renal failure following zoledronate treatment.9 In only three of these cases was there a history of pre-existing kidney disease. Given the lack of data concerning patients with impaired kidney function, it is unclear whether zoledronate should be used in CKD patients.
The severity of renal toxicity ranges from transient elevations in SCr to segmental glomerulosclerosis with signs of acute renal failure (i.e., proteinuria, dialysis need). In case reports involving renal toxicity following zoledronate exposure, toxicity developed after only a few infusions.10-13 A retrospective analysis examined patients with hormone-refractory prostate cancer with bone metastasis who had received at least 1 infusion of zoledronate.14 Of the 122 patients studied, 23.8% had renal impairment (defined as >25% decrease in creatinine clearance [CrCl]). The risk of renal impairment increased with duration of therapy (<6 months, 11.1%; >24 months, 26.3%), prior treatment with pamidronate, increasing age at therapy initiation, history of renal disease, hypertension, and cigarette smoking (P <.05).14
Zoledronate appears to cause nephrotoxicity in patients with normal kidney function; for this reason, its use in CKD patients cannot be justified.
Risedronate
Risedronate is indicated for the treatment of glucocorticoid-induced osteoporosis, osteoporosis in men, Paget’s disease, and postmenopausal osteoporosis. Risedronate is not recommended in patients with CrCl <30 mL/min.15
Some case reports have linked risedronate to renal toxicity. Ozkurt et al described a 54-year-old woman who presented with hemolysis and acute tubular necrosis after taking one 35-mg dose of risedronate.16 All laboratory values were within normal limits 3 days prior to exposure, and the patient had no pre-existing kidney disease or impaired renal function.
Other data suggest that risedronate may be safe for patients with moderate-to-severe kidney disease. Miller et al studied the safety of risedronate in women with decreased renal function by evaluating data from nine clinical trials.17 This meta-analysis included 9,996 patients (placebo, n = 4,500; risedronate 5 mg, n = 4,496) studied for an average of 2 years. All patients had renal impairment (CrCl <80 mL/min). In 48% of patients, renal impairment was mild (CrCl >50 to <80 mL/min); in 45% it was moderate (CrCl >30 to <50 mL/min); and in 7% it was severe (CrCl <30 mL/min). Median baseline CrCl was 49.5 mL/min for the placebo group and 49.2 mL/min for the risedronate group. The incidence of renal function–related adverse events (i.e., hematuria, hydronephrosis, kidney failure, acute kidney failure, abnormal kidney function, uremia, oliguria, polyuria, glomerulitis, nephritis) was similar within and between treatment groups.17 There were no statistically significant between-group differences as measured by SCr at 6, 12, and 24 months or at the endpoint (maximum 3 years). This suggests that risedronate 5 mg daily is a safe treatment for women with osteoporosis for up to 3 years, including patients with estimated CrCl <30 mL/min.
Kikuchi et al studied the effectiveness of risedronate and alfacalcidol in preventing osteoporosis resulting from long-term use of glucocorticoids to treat inflammatory renal diseases.18 Although baseline SCr and blood urea nitrogen (BUN) serum concentrations appeared to be normal, all patients had glomerulonephritis. Between-group differences in SCr and CrCl were evaluated at baseline and at the conclusion of the study. No differences in SCr and CrCl were found, and there appeared to be no adverse effects on kidney function.18 These findings agree with results reported by Fujii et al, who followed 114 subjects who had been taking glucocorticoids for chronic inflammatory kidney diseases for 1 year.19 CrCl was >30 mL/min in all subjects. At the end of the study, the mean change in CrCl was similar between groups. Risedronate had minimal to no adverse effects on kidney function.
Renal-transplant patients receiving long-term glucocorticoid treatment are at greater risk for bone deterioration because of pre-existing renal dysfunction. Torregrosa et al studied the use of bisphosphonates for treating osteoporosis and osteopenia in 84 renal-transplant patients (12-36 months posttransplantation).20 Patients were randomized to receive either risedronate 35 mg weekly plus vitamin D and calcium (cholecalciferol 800 IU + calcium carbonate [CaCO3] 2,500 mg daily, n = 39) or vitamin D and calcium only (n = 45). Risedronate was well tolerated, with no major adverse events or dropouts. SCr did not differ significantly between groups at baseline, 6 months, or 1 year after risedronate therapy, which suggests that risedronate has no severe adverse effects on renal function in posttransplant patients.
Alendronate
Alendronate is indicated for the treatment and prevention of postmenopausal osteoporosis, glucocorticoid-induced osteoporosis, osteoporosis in men, and Paget’s disease.21 Alendronate is eliminated unchanged by the kidney, and a dose adjustment is indicated at CrCl <35 mL/min. Wetmore et al studied the effects of short-term use of alendronate on bone mineral density (BMD) in hemodialysis patients.22 Thirty-one patients were assigned to receive either alendronate 40 mg weekly for 6 weeks (n = 16) or placebo (n = 15). No statistically significant differences in BUN or SCr were observed between groups at baseline or after 6 months. Three patients reported mild gastrointestinal upset, and no patient had to discontinue the study. These results indicated that alendronate was safe for patients receiving hemodialysis.
Jamal et al performed a data analysis of 581 women with reduced renal function (CrCl <45 mL/min) who were participating in the Fracture Intervention Trial (FIT).23 Patients were given alendronate 5 mg daily for 2 years; the dosage was increased to 10 mg daily after the second year owing to concurrent studies that found 10 mg to be more effective for building bone density.24 Treatment continued for another year in patients with pre-existing vertebral fractures, or for another 2 years in those without pre-existing fractures. Alendronate increased total hip BMD by 5.6% (95% CI 4.8-6.5) in patients with decreased CrCl, versus 4.8% (95% CI 4.6-5.0, P = .04) in those with normal CrCl.23 There was no difference in increases in BMD in the femoral neck or spine between patients with decreased CrCl and those with normal CrCl. There was no difference in reduction of risk of clinical fractures or vertebral fractures. Increases in SCr were the same in patients with decreased CrCl and those with normal CrCl (0.01 ± 0.10). From baseline to 3-year follow-up, SCr in both groups increased to 1.05 ± 0.16.23 Overall, alendronate was found to be safe and effective for the treatment of osteoporosis in women with decreased renal function. Trabulus et al studied the effects of alendronate in post–renal-transplant patients for up to 1 year and reported similar results.25
Yanik et al performed a comparison study of the renal safety of risedronate, alendronate, and raloxifene, all of which all are commonly used to manage osteoporosis in postmenopausal women.26 One hundred and twenty-seven patients diagnosed with osteoporosis or osteopenia were randomized to receive alendronate 70 mg once weekly (n = 47), risedronate 35 mg once weekly (n = 44), or raloxifene 60 mg per day (n = 36) for 1 year. No significant changes in renal function from baseline to study conclusion were noted.26
The studies by Wetmore, Trabulus, and Yanik had fairly small sample sizes, making it hard to generalize the findings to a larger, more diverse patient population. Jamal’s secondary analysis of the FIT, however, examined a larger patient population.23 Although the study did not include men, the design and dosing were strong. The study lasted 3 to 4 years (depending on patient demographics), which allowed for investigation of the long-term effects of alendronate. The alendronate dosage increased over the study period based on evidence from concurrent trials. This gave the study more applicability since it used clinically significant dosing. Regardless of sample size, the other three studies concluded that alendronate had minimal effects on existing renal function.
Etidronate
Etidronate is indicated for the treatment of Paget’s disease, heterotopic ossification, and hypercalcemia of malignancy.27 Etidronate is excreted unchanged by the kidney. Although the manufacturer recommends a dose reduction in patients with renal impairment, no defined dose adjustment is given. Etidronate should be used with caution when SCr is >2.5 mg/dL, and it is not recommended for patients with SCr >5 mg/dL.27 In a study of 141 patients with corticosteroid-induced osteoporosis, only 1 etidronate patient developed elevated SCr and withdrew from the study.28
Etidronate has been used in patients with renal dysfunction to prevent further cardiovascular calcification. Hashiba et al conducted a study to determine whether etidronate could effectively decrease aortic calcification in hemodialysis patients.29 Eighteen subjects were randomly assigned to the etidronate group (200 mg daily before bedtime [n = 8]) or the control group (n = 10) for 6 months. In an evaluation of etidronate’s safety, calcium ions (Ca2+), phosphate, Ca2+ x phosphate, and systolic blood pressure did not change. Hashiba et al conducted another study in 21 patients with aortic calcification receiving hemodialysis.30 Patients were initially observed for 12 months without therapy. During the next 23 months, 12 patients received etidronate 200 mg daily before bedtime, which produced similar results to the first study. Etidronate’s effects on kidney function cannot be accurately determined from these studies since the patients were receiving hemodialysis and likely did not have functioning kidneys at baseline. Oral etidronate use in CKD patients (stage 3/4) and its effect on existing renal function is inadequately documented. IV etidronate has been reported to be associated with acute renal failure.31,32 Data are insufficient at this time to conclude whether etidronate is safe in the CKD population.
Ibandronate
Ibandronate is indicated for the treatment and prevention of postmenopausal osteoporosis. Fifty percent to 60% of ibandronate is eliminated unchanged by the kidney. Ibandronate dosing does not need to be adjusted in patients with mild or moderate renal impairment; however, in patients with severe renal impairment (CrCl <30 mL/min), ibandronate is not recommended.33 Several studies have been conducted to assess the efficacy of ibandronate in patients with renal disease.
Bergner et al studied the effects of ibandronate in 16 patients with renal osteodystrophy and secondary hyperparathyroidism who were undergoing scheduled hemodialysis.34 After dialysis, a standard dose of ibandronate 2 mg IV was infused over 5 minutes every 4 weeks for 12 weeks. The study concluded that, with its ability to bind bone at such a high rate, ibandronate may effectively stop bone loss in patients with end-stage renal disease (ESRD) and secondary hyperparathyroidism. Bergner et al also studied the effect of ibandronate on 16 patients with ESRD and renal osteodystrophy over a 48-week period, with similar results.35 Despite small study sizes, these results showed ibandronate to be effective for decreasing bone turnover and increasing BMD in patients with ESRD. Other studies have found ibandronate to have a favorable renal-safety profile in patients with varying degrees of renal function.36,37
Pamidronate
Pamidronate is indicated for the treatment of osteolytic bone metastasis associated with multiple myeloma and metastatic breast cancer, Paget’s disease, and hypercalcemia due to malignancy.38 Pamidronate is eliminated completely by renal excretion. Dose adjustment is not necessary for patients with decreased CrCl; however, it is recommended that patients with any renal insufficiency not exceed a dose of 90 mg per month and that the drug be avoided in severe renal impairment. Prolonged doses greater than 90 mg monthly have resulted in cases of acute renal failure.39 Case reports also have noted acute renal failure in patients receiving standard-dose pamidronate infusions.40,41 Few studies have been performed examining the effects of pamidronate on patients with existing CKD.
Coco et al studied the effects of pamidronate on 72 patients who had received a renal transplant.42 The patients were randomized into two groups. One group received oral CaCO3 and calcitriol after the transplant; the second group received pamidronate 60 mg, CaCO3, and calcitriol 48 hours after the transplant and then again at months 1, 2, 3, and 6. SCr concentrations decreased significantly (P <.05) within the first 6 months in both groups, as would be expected immediately following a kidney transplant. SCr concentrations remained within normal limits (0.6-1.5 mg/dL as defined by the investigators) throughout the 12-month study period. These results suggest that pamidronate has no adverse renal effects.
Tiludronate
Tiludronate is indicated for the treatment of Paget’s disease. Its use is not recommended in patients with CrCl <30 mL/min.43 Pharmacokinetic data from 8 subjects with impaired renal function (CrCl 10-30 mL/min) given a single 400-mg dose of tiludronate indicate a 3 times larger AUC and a 5 times longer half-life than in subjects with normal renal function.44 When given at the indicated dose of 400 mg per day for 3 months, oral tiludronate appears to induce no major kidney-related adverse effects in patients with normal renal function.45 Higher doses and IV formulations from earlier studies appear to produce more nephrotoxic effects.46 Well-controlled studies investigating the use of tiludronate in large numbers of CKD or hemodialysis patients are not available. Tiludronate therefore is not recommended for the CKD population.
Selecting Appropriate Bisphosphonate Therapy for CKD Patients
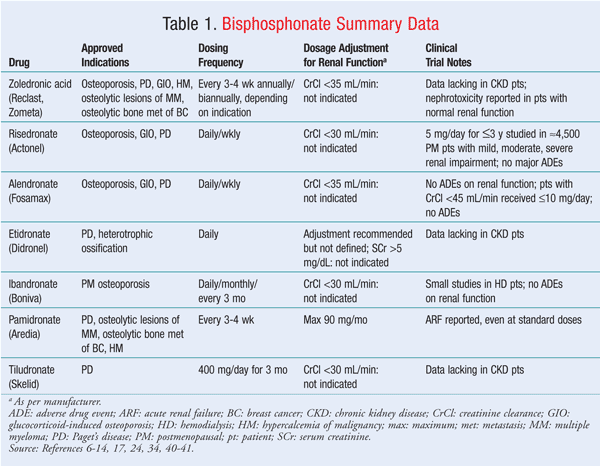
In general, large, randomized, double-blind, placebo-controlled trials evaluating bisphosphonate use in CKD patients are limited. Zoledronate has the propensity to produce nephrotoxicity in patients with normal renal function; it would seem logical that this would also occur in patients with impaired renal function, although no definitive trials directly support this assumption. Risedronate and ibandronate, which have a lower incidence of nephrotoxicity, have been studied in CKD patients with CrCl <30 mg/mL. Ibandronate has been studied in hemodialysis patients and was well tolerated, which suggests that this agent could be an option for patients with CKD and osteoporosis. Alendronate appears to be a viable agent, as it has been used in CKD patients at both 10 mg per day and 70 mg per week without adversely affecting renal function. Pamidronate can be used in patients with varying degrees of renal function as long as the dose does not exceed 90 mg per month, although there have been case reports of nephrotoxicity in patients receiving lower doses. Etidronate has been used in hemodialysis patients to delay cardiovascular calcifications. Significant data are not available for CKD patients not yet receiving hemodialysis. Finally, tiludronate, because of its extended half-life, expanded AUC, and lack of well-controlled studies in CKD, should be avoided in patients with impaired kidney function. TABLE 1 summarizes the bisphosphonates and their effects on renal function.
REFERENCES
1. Whaley-Connell AT, Sowers JR, McFarlane SI, et al. CKD in the United States: Kidney Early Evaluation Program (KEEP) and National Health and Nutrition Examination Survey (NHANES) 1999-2004. Am J Kidney Dis. 2008;51(suppl 2):S13-S20.
2. Looker AC, Orwoll ES, Johnston CC Jr, et al. Prevalence of low femoral bone density in older U.S. adults from NHANES III. J Bone Miner Res. 1997;12:1761-1768.
3. Siris ES, Miller PD, Barrett-Connor E, et al. Identification and fracture outcomes of undiagnosed low bone mineral density in postmenopausal women: results from the National Osteoporosis Risk Assessment. JAMA. 2001;286:2815-2822.
4. Reclast (zoledronic acid) product information. East Hanover, NJ: Novartis Pharmaceuticals Corp; May 2009.
5. Zometa (zoledronic acid) product information. East Hanover, NJ: Novartis Pharmaceuticals Corp; October 2009.
6. Saad F, Gleason DM, Murray R, et al. A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J Natl Cancer Inst. 2002;94:1458-1468.
7. Rosen LS, Gordon D, Tchekmedyian NS, et al. Long-term efficacy and safety of zoledronic acid in the treatment of skeletal metastases in patients with nonsmall cell lung carcinoma and other solid tumors: a randomized, Phase III, double-blind, placebo-controlled trial. Cancer. 2004;100:2613-2621.
8. Chang JT, Green L, Beitz J. Renal failure with the use of zoledronic acid. N Engl J Med.
9. Munier A, Gras V, Andrejak M, et al. Zoledronic acid and renal toxicity: data from French adverse effect reporting database. Ann Pharmacother. 2005;39:1194-1197.
10. Markowitz GS, Fine PL, Stack JI, et al. Toxic acute tubular necrosis following treatment with zoledronate (Zometa). Kidney Int. 2003;64:281-289.11. Joensuu TK. Renal toxicity following zoledronic acid reversed with ibandronate in a prostate cancer patient with bone metastases. Urol Int. 2008;80:448-450.
12. Bodmer M, Amico P, Mihatsch MJ, et al. Focal segmental glomerulosclerosis associated with long-term treatment with zoledronate in a myeloma patient. Nephrol Dial Transplant. 2007;22:2366-2370.
13. Aguiar Bujanda D, Bohn Sarmiento U, Cabrera Suárez MA, Aguiar Morales J. Assessment of renal toxicity and osteonecrosis of the jaws in patients receiving zoledronic acid for bone metastasis. Ann Oncol. 2007;18:556-560.
14. Oh WK, Proctor K, Nakabayashi M, et al. The risk of renal impairment in hormone-refractory prostate cancer patients with bone metastases treated with zoledronic acid. Cancer. 2007;109:1090-1096.
15. Actonel (risedronate sodium) product information. Cincinnati, OH: Procter & Gamble Pharmaceuticals, Inc; December 2009.
16. Ozkurt ZN, Güliter S, Keles I, Keles H. Risedronate-induced intravascular haemolysis complicated by acute tubular necrosis. Clin Rheumatol. 2005;24:665-666.
17. Miller PD, Roux C, Boonen S, et al. Safety and efficacy of risedronate in patients with age-related reduced renal function as estimated by the Cockcroft and Gault method: a pooled analysis of nine clinical trials. J Bone Miner Res. 2005;20:2105-2115.
18. Kikuchi Y, Imakiire T, Yamada M, et al. Effect of risedronate on high-dose corticosteroid-induced bone loss in patients with glomerular disease. Nephrol Dial Transplant. 2007;22:1593-1600.
19. Fujii N, Hamano T, Mikami S, et al. Risedronate, an effective treatment for glucocorticoid-induced bone loss in CKD patients with or without concomitant active vitamin D (PRIUS-CKD). Nephrol Dial Transplant. 2007;22:1601-1616.
20. Torregrosa JV, Fuster D, Pedroso S, et al. Weekly risedronate in kidney transplant patients with osteopenia. Transpl Int. 2007;20:708-711.
21. Fosamax (alendronate sodium) product information. Whitehouse Station, NJ: Merck & Co, Inc; March 2010.
22. Wetmore JB, Benet LZ, Kleinstuck D, Frassetto L. Effects of short-term alendronate on bone mineral density in haemodialysis patients. Nephrology (Carlton). 2005;10:393-399.
23. Jamal SA, Bauer DC, Ensrud KE, et al. Alendronate treatment in women with normal to severely impaired renal function: an analysis of the Fracture Intervention Trial. J Bone Miner Res.
24. Black DM, Thompson DE, Bauer DC, et al. Fracture risk reduction with alendronate in women with osteoporosis: the Fracture Intervention Trial. J Clin Endocrinol Metab. 2000;85:4118-4124.
25. Trabulus S, Altiparmak MR, Apaydin S, et al. Treatment of renal transplant recipients with low bone mineral density: a randomized prospective trial of alendronate, alfacalcidol, and alendronate combined with alfacalcidol. Transplant Proc. 2008;40:160-166.
26. Yanik B, Bavbek N, Yanik T, et al. The effect of alendronate, risedronate, and raloxifene on renal functions, based on the Cockcroft and Gault method, in postmenopausal women. Ren Fail.
27. Didronel (etidronate disodium) product information. North Norwich, NY: Norwich Pharmaceuticals, Inc; 2007.
28. Adachi J, Bensen W, Brown J, et al. Intermittent etidronate therapy to prevent corticosteroid-induced osteoporosis. N Engl J Med. 1997;337:382-387.
29. Hashiba H, Aizawa S, Tamura K, et al. Inhibitory effects of etidronate on the progression of vascular calcification in hemodialysis patients. Ther Apher Dial. 2004;8:241-247.
30. Hashiba H, Aizawa S, Tamura K, Kogo H. Inhibition of the progression of aortic calcification by etidronate treatment in hemodialysis patients: long-term effects. Ther Apher Dial. 2006;10:59-64.
31. Bounameaux H, Schifferli J, Montani J, et al. Renal failure associated with intravenous diphosphonates. Lancet. 1983;1:471-472.
32. O’Sullivan TL, Akbari A, Cadnapaphornchai P. Acute renal failure associated with the administration of parenteral etidronate. Ren Fail. 1994;16:767-773.
33. Boniva (ibandronate sodium) product information. South San Francisco, CA: Genentech USA, Inc; March 2010.
34. Bergner R, Henrich D, Hoffmann M, et al. High bone-binding capacity of ibandronate in hemodialysis patients. Int J Clin Pharmacol Res. 2005;25:123-131.
35. Bergner R, Henrich D, Hoffmann M, et al. Treatment of reduced bone density with ibandronate in dialysis patients. J Nephrol. 2008;21:510-516.
36. Pecherstorfer M, Diel IJ. Rapid administration of ibandronate does not effect renal functioning: evidence from clinical studies in metastatic bone disease and hypercalcaemia of malignancy. Support Care Cancer. 2004;12:877-881.
37. Bergner R, Henrich DM, Hoffmann M, et al. Renal safety and pharmacokinetics of ibandronate in multiple myeloma patients with or without impaired renal function. J Clin Pharmacol. 2007;47:942-950.
38. Aredia (pamidronate disodium) product information. East Hanover, NJ: Novartis Pharmaceuticals Corp; November 2008.
39. Markowitz G, Appel G, Fine P, et al. Collapsing focal segmental glomerulosclerosis following treatment with high-dose pamidronate. J Am Soc Nephrol. 2001;12:1164-1172.
40. Banerjee D, Asif A, Striker L, et al. Short-term, high-dose pamidronate-induced acute tubular necrosis: the postulated mechanisms of bisphosphonate nephrotoxicity. Am J Kidney Dis. 2003;41:E18.
41. Kunin M, Kopolovic J, Avigdor A, Holtzman EJ. Collapsing glomerulopathy induced by long-term treatment with standard-dose pamidronate in a myeloma patient. Nephrol Dial Transplant.
42. Coco M, Glicklich D, Faugere MC, et al. Prevention of bone loss in renal transplant recipients: a prospective, randomized trial of intravenous pamidronate. J Am Soc Nephrol. 2003;14:2669-2676.
43. Skelid (tiludronate disodium) product information. Bridgewater, NJ: sanofi-aventis U.S. LLC; April 2006.
44. Sansom LN, Necciari J, Thiercelin JF. Human pharmacokinetics of tiludronate. Bone.
45. Reginster JY, Treves R, Renier JC, et al. Efficacy and tolerability of a new formulation of oral tiludronate (tablet) in the treatment of Paget’s disease of bone. J Bone Miner Res. 1994;9:615-619.
46. Dumon JC, Magritte A, Body JJ. Efficacy and safety of the bisphosphonate tiludronate for the treatment of tumor-associated hypercalcemia. Bone Miner. 1991;15:257-266.
To comment on this article, contact rdavidson@uspharmacist.com.






