US Pharm. 2023;48(9):34-39.
ABSTRACT: The term transgender describes someone whose gender identity differs from the sex assigned at birth. Transgender persons often face challenges when seeking healthcare services. Treatment options for facilitating male-to-female and female-to-male gender transitions include behavioral therapy, medication therapy, and surgery. In addition to basic healthcare needs, transgender individuals may experience strains in interpersonal relationships and, potentially, mental-health issues related to gender incongruence. There are various considerations and monitoring parameters that healthcare providers should be aware of. Pharmacists can play an integral role in transgender patients’ care by providing comprehensive, gender-affirming medication management and other support as individuals move through their transition process.
In the United States, more than 1.6 million persons aged >13 years identify as transgender, part of the LGBTQIA+ community.1 Transgender describes an individual whose gender identity differs from the sex assigned at birth. Members of the transgender community often experience challenges when seeking healthcare services. Transgender individuals may opt to undergo hormone therapy (HT) or surgery. This article will discuss the clinical process for this transition and patient-care considerations for pharmacists.
Key Terminology
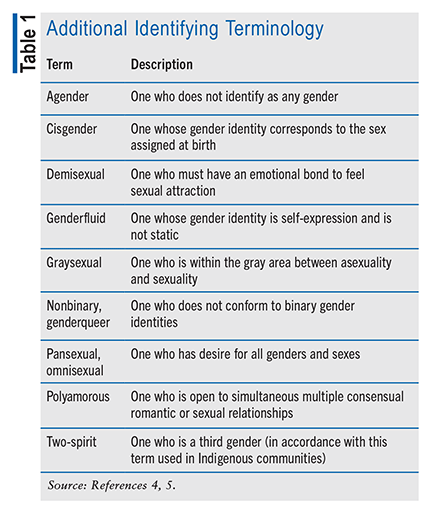
The letters in the acronym LGBTQIA+ represent the terms lesbian, gay, bisexual, transgender, queer/questioning, intersex, asexual, and “plus” (defined below).2 Lesbian refers to a woman who is attracted to other women. Gay describes someone who is attracted to persons of the same sex. Bisexual denotes a person who can be attracted to both women and men. Transgender, as noted earlier, is an umbrella term for individuals whose gender identity is different from that assigned at birth. The term queer is used by persons whose sexual orientation is not exclusively straight or heterosexual, whereas those who identify as questioning are contemplating their gender identity or sexual orientation. Intersex describes a person with one or more innate sex characteristics (e.g., internal reproductive organs, genitals, chromosome patterns) that are outside the traditional concepts of the female or male body. Asexual defines someone who does not feel sexual attraction and lacks the desire for romantic relationships. Plus represents orientations and identities yet to be described.2
Broadly speaking, transgender persons identify as or believe they are the opposite sex from that assigned at birth. Gender identity describes how the individual wants to identify to family, friends, and the public. Gender transition is when transgender persons begin to identify which sex they want to be or to be referred to; this could include changing their attire, name, or appearance—depending on how they want to be perceived—and undergoing HT. It is extremely important to always treat transgender individuals with equity and respect for their terminology. Gender expression is how the person appears externally, such as attire. Transgender individuals who do not want to identify as female or male may use other terms. TABLE 1 provides additional terminology.3-5
Transition Options
Male-to-Female: A transgender woman is an individual who was assigned male at birth and transitions to female by undergoing HT including estrogen.6 The most common method of transition involves SC injection of a gonadotropin-releasing hormone agonist (GNRH; TABLE 2) every 4 weeks with oral estrogen (17-beta-estradiol) for the 24 months leading up to sex-reassignment surgery. To enhance the estrogenic effects, antiandrogens are used to lower testosterone and minimize masculine sexual characteristics. Antiandrogens are also administered to decrease the occurrence of depression and hepatotoxicity.7 Dose-dependent effects include increased risks of venous thromboembolism, myocardial infarction, and stroke as well as increased prolactin levels.7 Transdermal and oral estradiol formulations are less likely to result in these side effects.
Female-to-Male: A transgender man is an individual who was assigned female at birth and transitions to male, primarily by undergoing HT with testosterone. The female-to-male process requires additional monitoring considerations. These patients must undergo physical examinations, such as breast and pelvic screenings; they also must be monitored every month after beginning testosterone therapy (TT) because of testosterone’s potential detrimental effects. TT can decrease insulin sensitivity and increase the patient’s lipid profile, liver enzymes, hematocrit, and weight. Patients receiving TT are at increased risk for heart disease, osteoporosis, and polycystic ovary syndrome.8 Common side effects include infertility, acne, obesity, increased libido, aggressiveness, and sleep apnea.8 To facilitate adherence, testosterone is offered in many formulations, such as transdermal patch, gel, and SC or IM injection. IM injection is the most common route of administration.
Pharmacokinetic Considerations
A patient’s sex and gender have an effect on drug safety and efficacy.9 This influence can be attributed to differences in body composition, kidney function, and metabolic activity.9 Body composition has an impact on drug disposition; however, data are limited regarding the effects that the HT used in transgender patients has on drug disposition. A meta-analysis found that in transgender men receiving testosterone, HT altered the body composition within 1 year of starting treatment; total body fat decreased and lean body mass increased during that time frame.10 Small cohort studies of patients receiving TT also showed a redistribution of regional fat.11,12 Visceral fat—which is typically higher in cisgender men than in cisgender women—increased, whereas abdominal fat decreased.11,12 Another study demonstrated an increase in total body fat and a reduction in lean body mass in transgender women undergoing estrogen therapy (ET).9 In small prospective studies, visceral and abdominal subcutaneous fat, as well as BMI, increased after ET initiation.12,13
Data on changes in drug-binding for the medications used in transgender patients are limited; however, some studies provide insight into the changes that occur in plasma protein concentrations while the patient is undergoing HT. Sex hormone-binding globulin (SHBG) was shown to decrease in transgender men receiving testosterone.14-16 Additionally, serum albumin was found to remain unchanged compared with baseline concentrations in testosterone-naïve patients, and transgender women receiving ET had a minimal reduction in serum albumin concentrations.16,17 Use of oral or injectable estradiol has been shown to result in increased SHBG concentrations.9
Data on sex- and gender-related differences in CYP metabolic activity are conflicting. However, predicted changes in drug-metabolizing proteins during HT have been established. ET in transgender women may inhibit some enzymes, such as CYP1A2 and CYP2C19, and increase the activity of UGT1A1, UGT1A4, and P-glycoprotein.9 Data on TT’s effect on those enzymes in transgender men are sparse.
Few studies have examined changes in kidney function and elimination in transgender adults. One prospective study reported increased serum creatinine concentrations throughout TT and a decrease during ET.18 Although data on long-term effects of HT on clearance in transgender adults are limited, it has been speculated that there would be no clinically significant changes.9
Medication Management
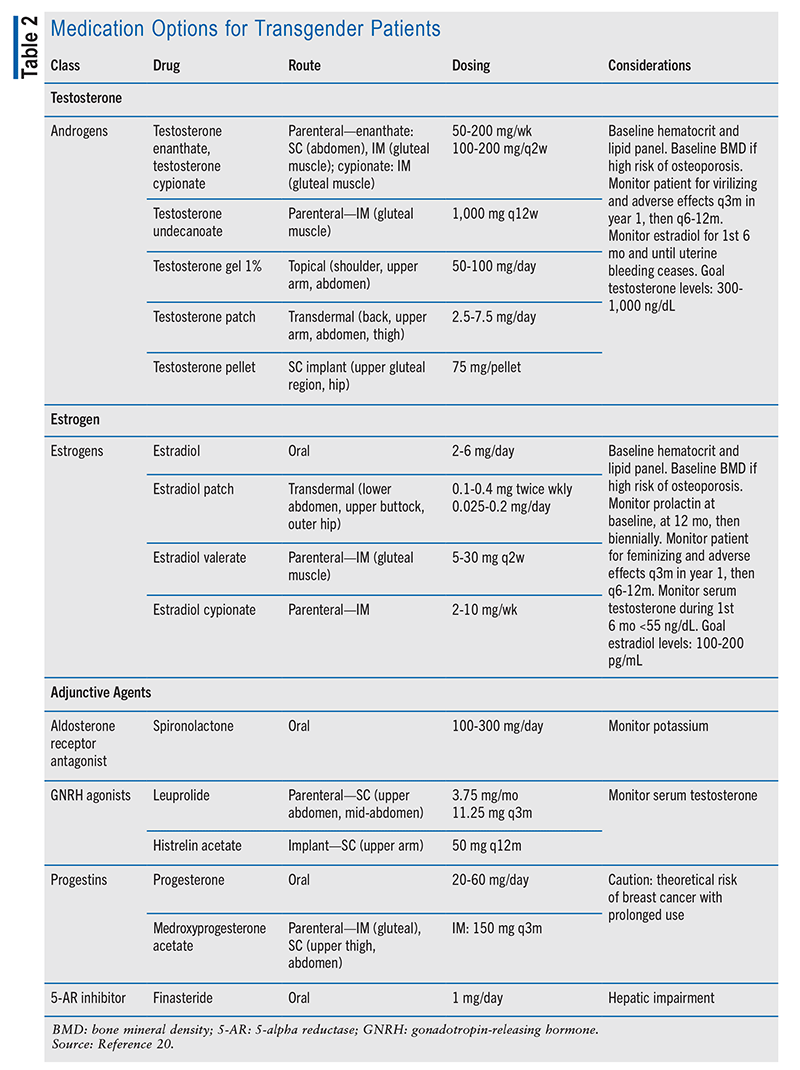
Clinicians use the Endocrine Society’s Endocrine Treatment of Gender-Dysphoric/Gender-Incongruent Persons clinical-practice guideline to determine the most appropriate regimen.19,20 According to the guideline, clinicians may prescribe TT or ET for transgender patients. Sex-hormone preparations are selected on an individual basis, taking into consideration the route of administration, price, and dosing. One of the standard treatments for transitioning patients is the use of cross-sex HT, which has been shown to have beneficial physical and psychological effects. Estrogen and antiandrogens are used to feminize transgender women while minimizing masculine features, whereas testosterone is utilized to reduce feminine characteristics in transgender men. TABLE 2 summarizes the available medication options for transgender patients.20
Transgender Men: TT formulations for transgender men include parenteral and transdermal routes. Testosterone enanthate and testosterone cypionate, which are parenteral, are dosed at 50 to 200 mg/week and 100 to 200 mg/10-14 days, respectively. Initial TT is dose dependent; higher doses may be used to sooner achieve adequate testosterone levels or physiologic effects. Testosterone gel 1% is dosed at 50 to 100 mg/day and the patch at 2.5 to 7.5 mg/day. Testopel pellets, which are implanted SC, are another option; dosed at 75 mg/pellet, this formulation releases testosterone slowly for a longer-lasting effect.20 TABLE 2 summarizes the available options.20
Testosterone dosing may be adjusted during the patient’s first year of HT to attain the desired results. Within 3 months of TT initiation, patients may experience amenorrhea; increased body hair, muscle mass, and libido; fat-distribution changes; and an increase in acne. Effects that may occur later include male pattern hair loss, vaginal-tissue atrophy, and deepening of the voice.20
Some of the laboratory parameters monitored in transgender men receiving TT are serum estradiol, serum total testosterone, serum free testosterone, and albumin. It is recommended to monitor for virilizing effects every 3 months during the first year of TT and every 6 to 12 months thereafter. Hematocrit and lipid profiles should be monitored upon initiation, then routinely based on the patient’s health status. Additionally, bone mineral density should be monitored in patients at risk for osteoporosis.
Transgender Women: Estrogen is the chief HT for transgender women.20,21 ET use in transgender women results in increases in breast tissue and body fat, decreased body-hair growth, and reduced testicular size; these effects may take up to 2 years to occur. Owing to its association with deep-vein thrombosis, ethinyl estradiol is no longer recommended for transgender patients. Preferred formulations include oral and transdermal estradiol and parenteral estradiol valerate. Dosing for oral estradiol is 2 to 6 mg daily, and that for transdermal estradiol is 0.1 to 0.4 mg twice weekly; estradiol valerate is dosed at 5 to 30 mg every 2 weeks. Dosing and titration are based on the patient’s response to therapy and hormone-level measurements.20,21
Adjunctive antiandrogens may be necessary to achieve adequate androgen suppression. Available agents include progesterone (20-60 mg orally daily); medroxyprogesterone acetate (150 mg IM every 3 months); GNRH agonists such as leuprolide (3.75 mg SC monthly or 11.25 mg SC every 3 months); histrelin implants (50 mg implanted every 12 months); finasteride (1 mg orally daily); and spironolactone (100-300 mg orally daily). TABLE 2 further summarizes the available formulations.20 One of the most frequently used medications for androgen suppression in transgender women is spironolactone, which is strongly associated with hyperkalemia and should be monitored. Other adverse-effect monitoring considerations include liver toxicity with finasteride use and breast cancer risk with long-term progestin use.20
Recommendations for routine monitoring in transgender women receiving ET are similar to those for transgender men receiving HT. During the first year, feminizing effects should be monitored every 3 months in transgender women, then every 6 to 12 months.20
Surgical Options
Procedures include facial feminization, transfeminine top, transfeminine bottom, facial masculinization, transmasculine top, and transmasculine bottom surgery. Facial feminization surgery and facial masculinization surgery alter the features of the patient’s face to appear feminine or masculine, respectively. Transfeminine top surgery enhances the shape and size of the breasts to create a more feminine chest appearance. Transfeminine bottom surgery is performed to reconstruct the male genitalia into female genitalia. In transmasculine top surgery, tissue is removed from the breasts to create a more masculine chest appearance. Transmasculine bottom surgery reconstructs the female genitalia into male genitalia.22
Mental-Health Considerations
Gender dysphoria (GD), which is defined as marked incongruence between an individual’s experienced/expressed gender and that assigned at birth, may occur in transgender persons.23 Signs and symptoms of GD include distress, anxiety, depression, self-image, and strong dislike of one’s sexual identity. In children, there is often a strong preference for the toys and activities associated with the opposite gender.Recent studies suggest that the prevalence of self-reported transgender identity in children, adolescents, and adults ranges from 0.5% to 1.3%.23,24
In GD, the individual cannot relate to traditional gender expression.23 This can cause strain in interpersonal relationships with family, friends, and peers and can affect aspects of daily life, potentially leading to depression and anxiety. Patients with GD may be treated with group counseling, HT, and surgery to achieve the desired physical appearance.23
Transgender persons experience disproportionate rates of depression, suicide ideation, and suicide attempts; this phenomenon has been linked to exposure to social stigma, gender-related victimization, and discrimination. One small cross-sectional study in Pakistan sought to quantify the prevalence of suicidal ideation in the transgender population and assess the relationship of depression to suicidal ideation.25 Factors analyzed relative to the presence of suicidal intent included age, family income, depression, smoking, and illicit substance use.25 In the study group of 156 transgender patients, 42.9% had suicidal ideation and 63.5% had depression according to the Hamilton Depression Rating Scale. With binary logistic regression, depression and illicit substance use were significantly related to the presence of suicidal ideation in the target population.25
Two longitudinal Chicago-area studies examined health-related issues in transgender youth and young adults: RADAR (N = 1,079, mean age 20.8 years) and FAB 400 (N = 488, mean age 19.57 years).26 The studies assessed health differences in self-reported health and related psychosocial variables between gender identities; they also compared all variables in transgender youth and cisgender sexual-minority peers from their cohort of origin. The study population comprised sexual and gender minorities who had been either assigned male at birth (AMAB) or assigned female at birth (AFAB). In total, there were 214 transgender participants (128 AFAB, 86 AMAB) across cohorts. Transgender youth had high rates of depression and suicidality, violence, and substance use, and transgender women and nonbinary AMAB youth reported worse health outcomes (except for depression) than transgender men and nonbinary AFAB youth.26 Findings point to the diversity of experiences within the transgender population and the critical need for interventional approaches to mitigate health disparities.26
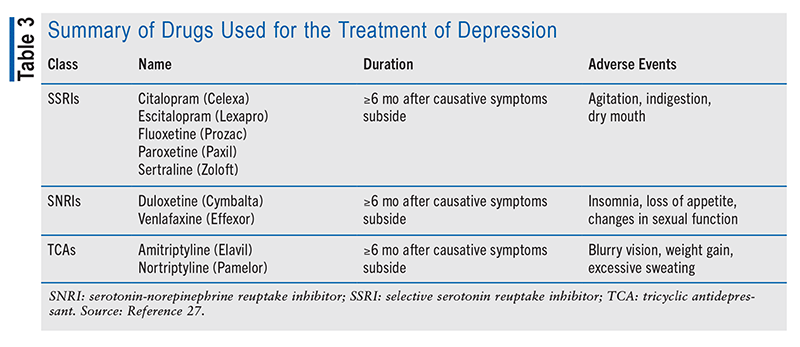
Nonpharmacologic and Pharmacologic Treatment: Patients should visit their primary care physician to discuss and initiate appropriate treatment. Treatment is determined by patient age; for example, group therapy that involves counseling on gender and HT might be beneficial for adolescents. Key components of counseling include a support team, a care team, and discussion points including realistic expectations, sexual health, and fertility preservation. Pharmacologic agents for treating depression include selective serotonin reuptake inhibitors, serotonin-norepinephrine reuptake inhibitors, and tricyclic antidepressants. These medications improve mood by blocking reuptake of serotonin alone or serotonin and norepinephrine. TABLE 3 outlines the medications in each drug class, including expected side effects.27
The Pharmacist’s Role
Because pharmacists have high patient accessibility, they are ideally positioned to assist transgender persons through unbiased, gender-affirming interactions. Specifically, pharmacists can play an integral role in the care of transgender patients by providing comprehensive, gender-affirming medication management and other support. Among the pharmacist’s responsibilities are appropriate laboratory monitoring and handling complex pharmacotherapeutic challenges.
Transgender persons have the same basic healthcare needs as their cisgender counterparts in terms of screening, prevention, and treatment; they also may have clinical issues specifically related to gender incongruence.28 Issues that pharmacists should be aware of include anxiety, depression, substance abuse, suicidal tendencies, and increased susceptibility to sexually transmitted diseases. Pharmacists can assist transgender patients and treatment teams by 1) increasing access to medications via pharmaceutical-industry programs and federal, state, and local agencies; 2) ensuring that the most cost-effective medications are dispensed; 3) discussing the most appropriate dosing regimens and formulations; 4) enumerating the risks and benefits of cross-sex HT; 5) carefully monitoring patients for efficacy and safety; and 6) suggesting risk-reduction strategies (e.g., smoking cessation, weight loss).29
Resources that pharmacists can provide to transgender patients in crisis include Trevor Project services (e.g., 24/7/365 Lifeline: 866-4-U-TREVOR [866-488-7386], online instant messaging [TrevorChat], text-based support [TrevorText], and online peer support [TrevorSpaces]); the National Suicide Prevention Lifeline (800-273-TALK [8255]); and the Trans Lifeline (877-565-8860).
Many transgender patients experience healthcare challenges that lead to disparities and barriers to receiving appropriate and culturally competent care. Pharmacists in various settings can play a crucial role in providing members of the transgender community with quality patient care.
REFERENCES
1. Herman JL, Flores AR, O’Neill KK. How Many Adults and Youth Identify as Transgender in the United States? Los Angeles, CA: The Williams Institute, UCLA School of Law; 2022.
2. The Lesbian, Gay, Bisexual & Transgender Community Center. What is LGBTQIA+? https://gaycenter.org/about/lgbtq/#+plus. Accessed January 21, 2023.
3. Zottola A. Transgender identity labels in the British press: a corpus-based discourse analysis. J Language Sexuality. 2018;7(2):237-262.
4. Betts J. What does LGBTQIA+ stand for? Full abbreviation and other terms explained. YourDictionary. https://abbreviations.yourdictionary.com/what-does-lgbtqia-stand-for-full-acronym-explained.html. Accessed January 21, 2023.
5. Merriam-Webster. Merriam-Webster’s short list of gender and identity terms. www.merriam-webster.com/wordplay/merriam-websters-short-list-of-gender-and-identity-terms. Accessed August 14, 2023.
6. Gooren LJ, Giltay EJ, Bunck MC. Long-term treatment of transsexuals with cross-sex hormones: extensive personal experience. J Clin Endocrinol Metab. 2008;93(1):19-25.
7. Jatenzo (testosterone undecanoate) product information. Buffalo Grove, IL: Tolmar, Inc; January 2023.
8. Depo-Testosterone (testosterone cypionate injection) product information. New York, NY: Pfizer Inc; August 2018.
9. Cirrincione LR, Huang KJ. Sex and gender differences in clinical pharmacology: implications for transgender medicine. Clin Pharmacol Ther. 2021;110(4):897-908.
10. Klaver M, Dekker MJHJ, de Mutsert R, et al. Cross-sex hormone therapy in transgender persons affects total body weight, body fat and lean body mass: a meta-analysis. Andrologia. 2017;49(5):e12660.
11. Elbers JM, Asscheman H, Seidell JC, et al. Long-term testosterone administration increases visceral fat in female to male transsexuals. J Clin Endocrinol Metab. 1997;82(7):2044-2047.
12. Elbers JM, Asscheman H, Seidell JC, Gooren LJ. Effects of sex steroid hormones on regional fat depots as assessed by magnetic resonance imaging in transsexuals. Am J Physiol. 1999;276(2):e317-e325.
13. Fisher AD, Castellini G, Ristori J, et al. Cross-sex hormone treatment and psychobiological changes in transsexual persons: two-year follow-up data. J Clin Endocrinol Metab. 2016;101(11):4260-4269.
14. Velho I, Fighera TM, Ziegelmann PK, Spritzer PM. Effects of testosterone therapy on BMI, blood pressure, and laboratory profile of transgender men: a systematic review. Andrology. 2017;5(5):881-888.
15. Haraldsen IR, Haug E, Falch J, et al. Cross-sex pattern of bone mineral density in early onset gender identity disorder. Horm Behav. 2007;52(3):334-343.
16. van Kesteren P, Lips P, Deville W, et al. The effect of one-year cross-sex hormonal treatment on bone metabolism and serum insulin-like growth factor-1 in transsexuals. J Clin Endocrinol Metab. 1996;81(6):2227-2232.
17. Chen H, Wiepjes CM, van Schoor NM, et al. Changes of vitamin D-binding protein, and total, bioavailable, and free 25-hydroxyvitamin D in transgender people. J Clin Endocrinol Metab. 2019;104(7):2728-2734.
18. Wierckx K, Van Caenegem E, Schreiner T, et al. Cross-sex hormone therapy in trans persons is safe and effective at short-time follow-up: results from the European Network for the Investigation of Gender Incongruence. J Sex Med. 2014;11(8):1999-2011.
19. Hembree WC, Cohen-Kettenis PT, Gooren L, et al. Endocrine treatment of gender-dysphoric/gender-incongruent persons: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2017;102(11):3869-3903.
20. Unger CA. Hormone therapy for transgender patients. Transl Androl Urol. 2016;5(6):877-884.
21. Deutsch MB. Overview of feminizing hormone therapy. UCSF Transgender Care. https://transcare.ucsf.edu/guidelines/feminizing-hormone-therapy. Accessed February 20, 2023.
22. American Society of Plastic Surgeons. Gender affirmation surgeries. www.plasticsurgery.org/reconstructive-procedures/gender-affirmation-surgeries. Accessed February 20, 2023.
23. Zucker KJ. Epidemiology of gender dysphoria and transgender identity. Sex Health. 2017;14(5):404-411.
24. Garg G, Elshimy G, Marwaha R. Gender dysphoria. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2023 Jan-.
25. Azeem R, Zubair UB, Jalil A, et al. Prevalence of suicide ideation and its relationship with depression among transgender population. J Coll Physicians Surg Pak. 2019;29(4):349-352.
26. Newcomb ME, Hill R, Buehler K, et al. High burden of mental health problems, substance use, violence, and related psychosocial factors in transgender, non-binary, and gender diverse youth and young adults. Arch Sex Behav. 2020;49(2):645-659.
27. VandenBerg AM. Depressive disorders. In: DiPiro JT, Yee GC, Haines ST, et al, eds. DiPiro’s Pharmacotherapy: A Pathophysiologic Approach. 12th ed. New York, NY: McGraw-Hill; 2023.
28. Redfern JS, Jann MW. The evolving role of pharmacists in transgender health care. Transgend Health. 2019;4(1):118-130.
29. Irving A, Lehault WB. Clinical pearls of gender-affirming hormone therapy in transgender patients. Ment Health Clin. 2018;7(4):164-167.
ACKNOWLEDGMENT: We extend heartfelt gratitude to our coauthor Paromita Naidu, BA, MA, MHA, for her invaluable contributions to this article. Her keen insights and dedication to addressing the critical need for increased awareness, research, and equity within the medical community for trans individuals were instrumental in shaping the direction and purpose of this work. Ms. Naidu’s thoughtful, open discussions about transgender health disparities spurred us to delve deeper into the multifaceted challenges this population faces when seeking healthcare services, especially within the context of pharmacy practice. Her passionate commitment to both promoting inclusivity and advocating for better healthcare outcomes in this marginalized community served as a continued motivation to us throughout the creation of this work.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.






