US Pharm. 2014;39(12):33-36.
ABSTRACT: Ebola virus disease, caused by the filovirus Ebolavirus, leads to viral hemorrhagic fever and is fatal in many cases. Outbreaks of Ebola in sub-Saharan Africa have resulted in fatality rates of up to 90%. The 2014 outbreak in West Africa is the largest epidemic to date. The virus enters the human chain through close contact with infected primates and other animals and can be passed human to human through contact with the bodily fluids of infected persons. Currently, neither a vaccine nor an effective antiviral treatment is available for use in humans. Preventive strategies and supportive therapy are the only options available for high-risk individuals and infected patients.
Ebola virus disease (formerly known as Ebola hemorrhagic fever) is caused by the Ebola virus.1 The genus Ebolavirus, along with Marburgvirus and Cuevavirus, is a member of the Filoviridae family (filoviruses or filamentous lipid-enveloped viruses).2 To date, five distinct species of Ebolavirus have been isolated: Sudan ebolavirus (SUDV), Zaire ebolavirus (EBOV), Tai Forest (Ivory Coast) ebolavirus (TAFV), Reston ebolavirus (RESTV), and Bundibugyo ebolavirus (BDBV).3
SUDV and EBOV first appeared in two simultaneous outbreaks in 1976: one in Sudan (now South Sudan), the other in a village near the Ebola River in the Democratic Republic of the Congo (formerly Zaire).4 In 1994, TAFV was associated with one fatality in Cote d’Ivoire.4 BDBV was discovered in a 2004 outbreak in Uganda. While RESTV has been found in humans in the Philippines and the People’s Republic of China, it has not been associated with any illness or deaths to date.1
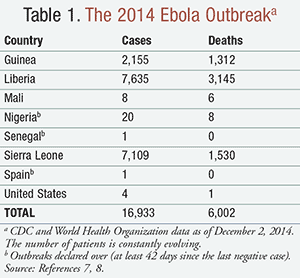
The 2014 outbreak is the largest epidemic to date, with the total number of cases exceeding those of all previous outbreaks combined.5 It has been attributed to EBOV and is the first outbreak of Ebola in West Africa (primarily Liberia, Sierra Leone, and Guinea).6 As of December 2014, there have been approximately 17,000 reported cases and 6,000 deaths (TABLE 1).7,8 A limited number of cases have occurred outside of West Africa. A nurse’s assistant in Spain was the first person outside Africa known to have contracted Ebola in the current outbreak.9 In the United States, two imported cases, including one death, and two secondary cases in healthcare workers have been reported.7 These cases have caused widespread public and government concern about Ebola.
Pathogenesis
The Ebola virus enters the human chain through close contact with the bodily fluids of primates and other animals, including chimpanzees, gorillas, fruit bats, monkeys, forest antelope, and porcupines found ill or dead in the rain forest.1 It can then be transmitted human to human through the mucous membranes, via tears in the skin, by direct contact with the infected person’s bodily fluids, or parenterally.1,10 The virus can also be spread by direct contact with the body of a deceased individual who was infected with the Ebola virus. Males can transmit the virus through their semen for up to 7 weeks following recovery.1 A natural host for the Ebola virus has not yet been identified; therefore, it is difficult to control the spread of the virus.11
The incubation period depends upon the route of infection, where parenterally infected individuals will show symptoms quicker than those infected by contact.10 The virus travels from the site of infection to the regional lymph nodes, liver, spleen, and adrenal glands. In the human body, the virus can infect monocytes, macrophages, dendritic cells, endothelial cells, fibroblasts, hepatocytes, adrenal cortical cells, and epithelial cells. Although the lymphocytes are not infected, they undergo apoptosis and therefore their numbers are reduced.10
It has been demonstrated that both the host and the viral proteins contribute to the pathogenesis of the Ebola virus.11 The virus seems to trigger the release of inflammatory cytokines, interferon (IFN)-gamma, IFN-lpha, interleukin-2 (IL-2), IL-10, and tumor necrosis factor (TNF)-alpha, which leads to subsequent vascular leak and clotting, ultimately resulting in multiorgan failure and shock.10,12
Hepatocellular necrosis, combined with massive viremia, leads to dysregulation of clotting factors and disseminated intravascular coagulopathy.10,11 In some cases, the adrenal cortex is affected, leading to hypotension and impaired steroid synthesis.10
Diagnosis
Diagnosis of the infection includes a physical examination and patient history. The African-derived Ebola virus infection has a typical incubation period of 9 to 11 days with symptoms appearing anywhere between 2 and 21 days after exposure to the virus.10 The symptoms will vary depending upon the stage of the disease.
In the early stages, infected patients present with flulike symptoms, including a fever, headache, joint and muscle aches, and weakness. As the infection progresses, patients may experience diarrhea, vomiting, stomach pain, and lack of appetite.13 Upon examination, pharyngitis, a maculopapular rash, gastrointestinal (GI) bleeding, hematologic irregularities, and bilateral conjunctival injection may be found.3 If the disease has progressed, a physical examination may reveal expressionless facies, bleeding from IV puncture sites and mucous membranes, myocarditis, and pulmonary edema. Terminally ill patients have been found to experience tachypnea, hypotensive shock, anuria, and coma.3
The mortality rate for the current outbreak has been about 40%, which is lower than that of past outbreaks.7,8 While infection with the Ebola virus is fatal in many patients, some do survive. In these individuals, recovery is a slow process, often taking months before the person can resume full activities. Long-term complications in survivors include myalgias, asymmetric and migratory arthralgias, headache, fatigue, bulimia, amenorrhea, hearing loss, tinnitus, unilateral orchitis, suppurative parotitis, and ocular disorders.3,14 A study conducted on the survivors of the 1995 Ebola outbreak in the Democratic Republic of the Congo showed that 15% of the survivors complained of ocular pain, photophobia, hyperlacrimation, and loss of visual acuity. All of these patients were successfully managed with topical 1% atropine and steroids.14
The patient’s history may reveal primary or secondary exposure to an individual infected with the Ebola virus. Primary exposure constitutes travel to an Ebola-endemic area, whereas secondary exposure constitutes human-to-human or primate-to-human exposure.3 Persons who have worked in tropical African forests in endemic areas, and to a lesser extent those who have lived within the cities of the same areas, are considered as having primary exposure to the virus. Those who care for infected patients or infected primates have a history of secondary exposure to the virus.
Rapid differentiation from other diseases such as malaria, typhoid fever, shigellosis, cholera, leptospirosis, plague, rickettsiosis, relapsing fever, meningitis, hepatitis, and other viral hemorrhagic fevers is critical for timely implementation of public health measures.1,15 The virus can be detected through a series of laboratory tests, including basic blood tests and serologic testing.
Basic blood tests include a complete blood count (CBC) with differential, bilirubin, liver enzymes, blood urea nitrogen, creatinine, and pH.3 Typical findings in patients infected with Ebola include leukopenia, in many cases with lymphopenia (lymphocytopenia).7 As the condition advances, elevated neutrophils and a left shift may be found.10 Platelet counts fall to between 50,000 and 100,000, and amylase and hepatic transaminase levels are elevated. Proteins may be found in the urine, prothrombin and partial thromboplastin times are prolonged, and fibrin degradation products are elevated.10 Serologic tests, including the enzyme-linked immuno-sorbent assay (ELISA) for antigens or for immunoglobulin M (IgM) and immunoglobulin G (IgG) antibodies and a reverse transcriptase-polymerase chain reaction (RT-PCR) assay, are useful in identifying the virus if the patient survives long enough to develop an immune response.3,15
Prevention of Ebola
Currently, there is no FDA-approved Ebola vaccine available, although the National Institute of Allergy and Infectious Diseases (NIAID) as well as other centers are working on developing vaccines against the disease.1 In the meantime, a number of preventive actions should be taken. The virus can be killed by a variety of disinfectants, including most handwashes and hypochlorite bleach.3
In healthcare settings, the focus should be on improving and maintaining high standards of infection control. Healthcare workers may unknowingly come into contact with patients suffering from Ebola since the initial symptoms are nonspecific to the disease. To avoid the possibility of coming into contact with an infected individual’s bodily fluids, healthcare workers must follow standard precautions at all times. These consist of the use of personal protective clothing and equipment including a face shield, medical mask, goggles, double gloves, and a clean, nonsterile, long-sleeved, fluid-impermeable gown. Furthermore, healthcare professionals should follow basic hand hygiene, respiratory hygiene, safe injection practices, effective waste disposal, and safe burial practices.1,16 Similarly, laboratory workers should be advised to handle patient samples with caution.
Communities should be educated on the nature of the disease, and necessary support should be provided. People should be advised to avoid direct or close physical contact with infected patients. Those caring for infected persons at home should be trained on the correct use of protective equipment and other protective strategies such as the regular washing of hands. Communities also need to be educated on safe burial practices, in many cases with the modification of long-standing cultural funeral practices.1,5
The consumption of bush meat and contact with bats should be discouraged. This can be achieved through socioeconomic development and education. In cases where the consumption of bush meat cannot be stopped immediately, safe slaughtering and handling methods should be taught to prevent the virus from entering the human chain.5
Experimental Vaccines
The NIAID and GlaxoSmithKline have codeveloped an experimental vaccine against the Ebola virus that has passed preclinical trials.17 It is being fast-tracked for phase I clinical trials in the U.S. A parallel trial is being conducted by the National Institutes of Health (NIH) and a British-based consortium (including the Wellcome Trust and Britain’s Medical Research Council and Department for International Development) to test the same vaccine in healthy volunteers in the United Kingdom. This trial was extended to Mali and Gambia in late September 2014. The vaccine, which is based upon a chimpanzee cold virus known as the chimp adenovirus type 3 (ChAd3), delivers a part of the genetic material of the Ebola virus to the vaccine recipient. Here the gene prompts an immune response in the vaccinated individual.17
The NIH has also partnered with the NewLink Genetics Corporation to develop another highly advanced vaccine that is based upon the vesicular stomatitis virus (VSV-EBO). Trials for this vaccine began in the U.S. (Maryland) in late 2014.17 Other vaccines currently being tested include one based upon the established rabies vaccines, and others are being developed by Crucell (a subsidiary of Johnson & Johnson) and Profectus BioSciences.18
Management
At the time this article was written, there were no agents available to treat the Ebola virus. Patient care is therefore centered on supportive care and strict isolation. Several management and supportive care strategies have been attempted in outbreaks, but none of them have been implemented or documented sufficiently to evaluate the efficacy of treatment.19 Due to the pathology of the virus, patients are frequently dehydrated with disrupted electrolyte balances.18 Generally, supportive therapy includes assessment and maintenance of intravascular volume, electrolytes, nutrition, and comfort.3 Most cases slowly progress towards multiorgan failure, shock, and eventual death, while some individuals may recover.18
Since survivors can produce infectious virions for prolonged periods, strict barrier isolation in a private room is vital.19 Any objects that come into contact with the infected patient or the patient’s bodily fluids should be disinfected with a 0.5% sodium hypochlorite solution. Deceased patients should be buried promptly with minimal contact.3
Unfortunately, all agents being developed for the treatment or prevention of the Ebola virus were under investigation at the time of the epidemic crisis.20 With pressure from the public, investigators are faced with the challenge of whether and how they can use these investigative therapies to reduce the number of deaths in the current epidemic. Clearly, this scenario raises a host of ethical questions, including whether investigative drugs should be administered to patients on a compassionate basis, how to select patients who are to receive the investigative drugs, and how the effects of these drugs should be monitored.20
The highlight of this debate is the agent known as ZMapp, which has been preliminarily tested by Mapp Biopharmaceuticals. ZMapp is an experimental monoclonal antibody-based therapy that has presently been tested only in animals.18 It is composed of three “humanized” monoclonal antibodies manufactured in the plant nicotiana. It is manufactured by combining the best components of MB-003 (Mapp) and ZMAb (Defyrus/PHAC).21
Two patients were successfully treated in the U.S. with ZMapp, but a priest in Spain who also received ZMapp subsequently died. Unfortunately, ZMapp was only identified as a drug candidate in January 2014 and, therefore, very small amounts of the drug are available.18
A variety of other drug targets are currently being investigated by several research groups. One study group is focusing on the Ebola virus matrix protein VP40. Only seven genes are encoded by the Ebola genome. These mediate the entry, replication, and egress of the virus from the host cell.2 Entry into the host cell is facilitated by the surface glycoprotein, most likely through receptor-mediated endocytosis.22 The viral fusion protein mediates subsequent fusion of viral and cellular membranes, and replication occurs in the cytoplasm. New viral particles are assembled at the plasma membrane.22 The matrix protein, VP40, which is found localized under the lipid envelope of the virus, plays an important role in the assembly of new virus particles and their budding prior to egress.2,22 A number of studies have shown that deletions or mutations in VP40 can block the process of viral egress; however, how this can be achieved using pharmacologic molecules is yet to be determined.2
The drug TKM-Ebola, developed by Tekmira Pharmaceuticals Corporation, is currently undergoing phase I clinical trials. It uses an interfering RNA molecule to silence the expression of two genes required for the replication of the Ebola virus.23
There are a number of other agents that are being studied for the management of the Ebola virus, focusing on different aspects of the Ebola virus pathophysiology.10 However, these are not as far along in the trial process as ZMapp and TKM-Ebola.
One research group has found that the drug molecule T-705 suppresses the replication of EBOV.24 When administered to mice, T-705 induced rapid virus clearance, reduced the biochemical parameters of disease severity, and prevented a lethal outcome in 100% of the animals.24
Conclusion
Pharmacists can play a large role in the management of the Ebola virus by educating and reassuring the public, particularly those traveling to endemic areas (TABLE 2 lists a summary of prevention strategies).13,25 As healthcare professionals, pharmacists are suitably placed to advise the general public on what measures can be taken to minimize the risk of infection, what symptoms to watch out for, and how to seek medical advice if contact is made with the Ebola virus.

REFERENCES
1. Ebola virus disease. World Health Organization. Updated September 2014. www.who.int/mediacentre/factsheets/fs103/en/. Accessed September 10, 2014.
2. Stahelin RV. Could the Ebola virus matrix protein VP40 be a drug target? Expert Opin Ther Targets. 2014;18(2):115-120.
3. King JW. Ebola virus infection. http://emedicine.medscape.com/article/216288-overview. Accessed September 9, 2014.
4. Towner JS, Sealy TK, Khristova ML, et al. Newly discovered ebola virus associated with hemorrhagic fever outbreak in Uganda. PLoS Pathog. 2008;4(11):e1000212.
5. Frieden TR, Damon I, Bell BP, et al. Ebola 2014—new challenges, new global response and responsibility. N Engl J Med. 2014;371(13):1177-1180.
6. Dixon MG, Schafer IJ. Ebola viral disease outbreak—West Africa, 2014. MMWR. Morb Mortal Wkly Rep. 2014;63(25):548-551.
7. 2014 Ebola outbreak in West Africa. CDC. Updated November 28, 2014. www.cdc.gov/vhf/ebola/outbreaks/2014-west-africa/index.html. Accessed December 2, 2014.
8. 2014 Ebola outbreak in West Africa—case counts. CDC. Updated December 2, 2014. www.cdc.gov/vhf/ebola/outbreaks/2014-west-africa/case-counts.html. Accessed December 2, 2014.
9. Ebola fast facts. CNN. November 1, 2014. www.cnn.com/2014/04/11/health/ebola-fast-facts/. Accessed November 6, 2014.
10. Ebola virus disease information for clinicians in U.S. healthcare settings. CDC. November 6, 2014. www.cdc.gov/vhf/ebola/hcp/clinician-information-us-healthcare-settings.html. Accessed November 6, 2014.
11. Sullivan N, Yang ZY, Nabel GJ. Ebola virus pathogenesis: implications for vaccines and therapies. J Virol. 2003;77(18):9733-9737.
12. Villinger F, Rollin PE, Brar SS, et al. Markedly elevated levels of interferon (IFN)-gamma, IFN-alpha, interleukin (IL)-2, IL-10, and tumor necrosis factor-alpha associated with fatal Ebola virus infection. J infect Dis. 1999;179(suppl 1):S188-S191.
13. Signs and symptoms. Ebola (Ebola virus disease). CDC. November 2, 2014. www.cdc.gov/vhf/Ebola/symptoms/index.html. Accessed November 6, 2014.
14. Kibadi K, Mupapa K, Kuvula K, et al. Late ophthalmologic manifestations in survivors of the 1995 Ebola virus epidemic in Kikwit, Democratic Republic of the Congo. J Infect Dis. 1999;179(suppl 1):S13-S14.
15. Leroy EM, Baize S, Lu CY, et al. Diagnosis of Ebola haemorrhagic fever by RT-PCR in an epidemic setting. J Medical Virol. 2000;60(4):463-467.
16. Ebola: protection of health workers on the front line. Lancet. 2014;384(9942):470.
17. NIH to launch human safety study of Ebola vaccine candidate. National Institute of Allergy and Infectious Diseases (NIAID). August 28, 2014. www.niaid.nih.gov/news/newsreleases/2014/Pages/EbolaVaxCandidate.aspx. Accessed September 14, 2014.
18. Hampton T. Largest-ever outbreak of Ebola virus disease thrusts experimental therapies, vaccines into spotlight. JAMA. 2014;312(10): 987-989.
19. Clark DV, Jahrling PB, Lawler JV. Clinical management of filovirus-infected patients. Viruses. 2012;4(9):1668-1686.
20. Joffe S. Evaluating novel therapies during the ebola epidemic. JAMA. 2014;312(13):1299-1300. Accessed September 13, 2014.
21. ZMapp information sheet. Mapp Biopharmaceutical, Inc. August 12, 2014. www.mappbio.com/zmapinfo.pdf. Accessed September 14, 2014.
22. Dessen A, Volchkov V, Dolnik O, et al. Crystal structure of the matrix protein VP40 from Ebola virus. EMBO J. 2000;19(16):4228-4236.
23. TKM-Ebola. Tekmira Pharmaceuticals Corporation. 2014. www.tekmira.com/pipeline/tkm-ebola.php. Accessed September 16, 2014.
24. Oestereich L, Ludtke A, Wurr S, et al. Successful treatment of advanced Ebola virus infection with T-705 (favipiravir) in a small animal model. Antiviral Res. 2014;105:17-21.
25. Prevention. Ebola (Ebola virus disease). CDC. November 5, 2014. www.cdc.gov/vhf/ebola/prevention/index.html. Accessed November 6, 2014.





