US Pharm. 2007;32(4):HS5-HS12.
Bacterial skin and soft tissue infections (SSTIs) collectively refer to several microbial invasions of the skin layers (epidermis, dermis, and subcutaneous tissues), inducing a host response. Infections may present either as cutaneous abscesses with a collection of pus surrounded by an area of erythema and swelling or as diffuse, spreading infections, as in cellulitis or erysipelas. Bacterial skin and soft tissue infections occur frequently and range in severity from superficial infections of mild to moderate severity to deeper, occasionally necrotizing, infections. 1 Although SSTIs represent a common indication for antibiotic therapy, an etiologic diagnosis is often difficult and most patients are treated empirically.2
As the description implies, complicated SSTIs are usually more severe, progress rapidly, involve deeper tissues, and possess a greater risk of limb loss than uncomplicated SSTIs. Complicated cellulitis, complex abscesses, posttraumatic or surgical site infections, and infected diabetic and ischemic ulcers are commonly encountered examples. Hospitalization along with surgical debridement or drainage, parenteral antibiotic therapy, and management of comorbid conditions such as peripheral vascular disease or diabetes mellitus is usually necessary.2,3
Epidemiology
Most skin and soft tissue infections are uncomplicated, of mild to modest
severity, and can be treated with oral antibiotics on an outpatient basis;
however, an estimated 10% of all hospital admissions are for the management of
SSTIs.3 In 1995, an estimated 330,000 Medicare patients in the
United States required hospitalization and treatment of SSTIs.4 In
addition, postoperative surgical site infections result in approximately
500,000 infections per year.3
Pathophysiology
Skin is composed of several layers and serves as a primary barrier against
infections in healthy individuals. Several factors may contribute to an
increased risk of infection, including damage to the outermost layer of skin,
high concentrations of bacteria, and inadequate blood supply.2
Infection occurs when virulence factors of the infecting organism overcome the
host's natural immune system. Subsequent invasion and dissemination of
microorganisms in viable tissue trigger a series of local and systemic host
responses.5 A variety of patient factors may contribute to skin
infections, including diabetes mellitus, peripheral vascular insufficiency or
neuropathy, disrupted lymphatic drainage, or surgical trauma.
Classification
There is no universally accepted classification scheme for SSTIs, which makes
comparison of medications or studies difficult. Primary infections occur in
otherwise healthy skin and are usually caused by Staphylococcus aureus
or Group A streptococci. Secondary infections usually occur as a result of
predisposing conditions that serve as portals of entry for bacteria. Bacterial
SSTIs can be further classified into complicated and uncomplicated infections.
3,6 As defined by FDA, complicated soft tissue infections involve
abnormal skin or wounds, infection in an immunocompromised patient or
infections requiring surgical intervention.7
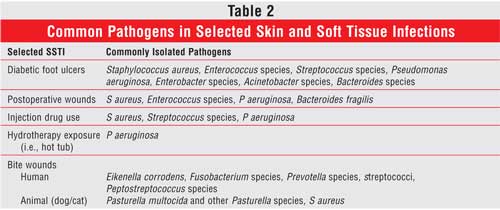
Microbiology
S aureus and streptococci are the predominant organisms responsible for
most SSTIs (Table 1). Streptococci are often classified into groups,
and those most commonly associated with SSTIs fall into groups A and B. Group
A streptococci (Streptococcus pyogenes) are often associated with
necrotizing fasciitis or "flesh-eating" infections. In diabetic patients,
Group B streptococci (Streptococcus agalactiae) are often causative
agents. Other organisms may be pathogenic under specific circumstances (
Table 2). Infected surgical sites, wounds due to trauma, and chronic
wounds are often caused by a mixture of aerobic and anaerobic micro-organisms.
Pseudomonas aeruginosa is often isolated from lower extremity infections,
particularly in cases with peripheral vascular disease or puncture wounds and
in cases involving hydrotherapy.1-3,6,8

Methicillin-resistant S aureus(MRSA)has been problematic for hospitals for decades, but an increasing incidence of community-acquired MRSA (CA-MRSA) cases has created a serious problem in treating SSTIs. The prevalence of methicillin-resistance in community-acquired S aureus in the U.S. is believed to be at least 20% and may be as high as 50% in some areas.9 Fortunately, most CA-MRSA isolates are susceptible to several oral antimicrobials including trimethoprim-sulfamethoxazole, doxycycline, and clindamycin, whereas hospital-acquired MRSA strains are not. Macrolide resistance among group A and group B streptococci has also risen, but organisms have remained susceptible to penicillins and cephalosporins. Changes in antimicrobial resistance patterns with S aureusand S pyogenescreate challenging clinical scenarios, since both of these organisms are primary causes of various SSTIs.
Diagnosis
A thorough physical examination is crucial to determine the severity of the
infection. Local signs that would suggest a serious SSTI include bullae,
hemorrhagic lesions, and numbness or pain. Extreme pain out of proportion to
physical findings, tenderness, or numbness around a wound is often associated
with tissue necrosis. Hypotension, tachycardia, hypothermia or fever, and
altered mental status are systemic symptoms suggestive of a more serious
infection.1-3,6,8 Presence of two or more of these signs is usually
associated with worse outcomes. Other determinants of the need for
hospitalization and surgical consultation are the size and location; lesions
located in the groin area, fingers and toes, and head are more likely to be
complicated.8
Unlike most uncomplicated infections where cultures of the skin rarely yield a definitive pathogen, gram staining and culture specimens obtained from complicated wounds generally help guide therapy, especially when MRSA is a suspected pathogen. Tissue specimens obtained by biopsy or curettage are preferred to swabs of superficial skin lesions or drainage, which may be contaminated with normal skin flora. If there is no open wound, needle aspiration of fluid at the leading edge of infection may be helpful. Although the yield is very low in complicated skin and soft tissue infections (cSSTIs), patients who present with severe infection or sepsis syndrome should have blood cultures obtained. Other blood tests may be helpful for diagnosis, including abnormal findings such as elevated white blood cell count, anemia, and thrombocytopenia. Some cases may warrant additional tests, such as x-rays, ultrasound, or computed tomography, to look for foreign bodies or collections of fluid. In cases where fasciitis or osteomyelitis is suspected, magnetic resonance imaging is often beneficial.
Nonpharmacologic Therapy
Surgical incision and drainage is a mainstay in the treatment of cSSTIs such
as abscesses. This process removes purulent material within the abscess as
well as a large portion of the microbes causing the infection. Hyperbaric
oxygen is also believed to enhance wound healing, although its use is
controversial. In theory, higher oxygen concentrations in the infected tissue
enhance eradication of infecting organisms and promote healing.10
Pharmacologic Therapy
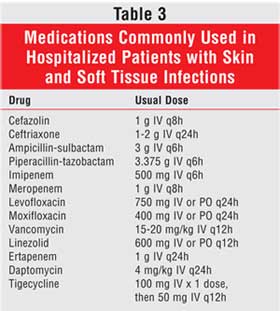
Several antibiotic classes have been shown to be effective, alone or in
combination, for treating cSSTIs (Table 3). Cephalosporins of all
generations (e.g., cefazolin, cefoxitin, ceftriaxone),
penicillin-beta-lactamase inhibitor combinations (e.g., ampicillin-sulbactam,
piperacillin-tazobactam), fluoroquinolones (e.g., levofloxacin, moxifloxacin,
ciprofloxacin), glycopeptides (e.g., vancomycin), streptogramins (e.g.,
quinupristin/dalfopristin),11,12 and carbapenems (e.g., imipenem,
meropenem) have been used successfully with reported clinical and
microbiological response rates usually between 70% and 80%. Clindamycin or
metronidazole can be added to a regimen to cover for anaerobes, depending on
the clinical situation and spectrum of other agents being used. Several
medication-related factors are important to consider when selecting an
antimicrobial agent, including the route of delivery, patient allergies, side
effect profile, drug interaction potential, pharmacodynamics, and cost.
The choice between parenteral and oral therapy is generally determined by the severity of the infection. In most cases parenteral therapy is preferred, at least initially, to ensure rapid serum and tissue antimicrobial levels. Dosing considerations are primarily influenced by the minimum inhibitory concentration (MIC) of the infecting organism and the drug concentration in tissues and body fluids. Patient factors, such as body weight and renal/hepatic function, should also be considered. With increasing resistance and number of MRSA infections, several novel antibiotics have been evaluated for the treatment of cSSTIs. The following section will discuss each agent and its place in therapy.
Linezolid: Linezolid, an oxazolidinone, inhibits bacterial growth by binding to the 50S ribosomal subunit preventing the formation of the 70S initiation complex and protein synthesis.13-15 Linezolid is active against many gram-positive organisms including S aureus (MRSA and methicillin-sensitive S aureus [MSSA]), coagulase-negative staphylococci, vancomycin-susceptible and vancomycin-resistant Enterococcus faecium and Enterococcus faecalis, and streptococci (including penicillin-resistant strains). Usually bacteriostatic, linezolid has been shown to be bacteriocidal against some strains of Streptococcus pneumoniae and S pyogenes.16 FDA-approved indications for linezolid include vancomycin-resistant E faecium infections, nosocomial pneumonia, skin and skin structure infections, and community-acquired pneumonia. Linezolid, 600 mg b.i.d. intravenous or oral, is approved for the use of cSSTI without osteomyelitis caused by S pyogenes, S agalactiae, and MSSA or MRSA.13,16
Several studies have compared linezolid, intravenous and oral, against standard therapy for the treatment of cSSTIs. One small study of hospitalized patients with MRSA cSSTI compared oral linezolid to intravenous vancomycin and found higher clinical cure and improvement rates in the patients that received oral linezolid.14 A second study compared linezolid and vancomycin for cSSTI and found similar results.15 Researchers found intravenous or oral linezolid to be as efficacious as oxacillin with switch to oral dicloxacillin for the treatment of cSSTI.17
Generally well tolerated, the most common adverse events documented for linezolid involved the digestive system: nausea (4.1%, 5.8%)15,17 and diarrhea (5.2%).15 Thrombocytopenia has been reported with long-term use of linezolid and has been a cause of discontinuation of linezolid therapy in studies. The convenience of an oral dosage form with 100% bioavailability gives linezolid a distinct advantage over standard therapy without an oral equivalent, especially in cases involving MRSA. Another advantage of using linezolid is the ability to continue therapy as an outpatient, thus decreasing the length of stay and overall cost. Although it may be given as first-line therapy, linezolid is best used for transitioning of care to outpatient status and as an alternative to standard therapy.
Ertapenem: Ertapenem, a 1-beta-methyl carbapenem, exhibits bacteriocidal activity by binding to specific bacteria penicillin-binding proteins (PRBPs) that interfere with cell wall synthesis. With a spectrum of activity similar to other carbapenems, it is active against gram-positive, gram-negative, and anaerobic bacteria such as S aureus (MSSA), S pyogenes, S pneumoniae, Escherichia coli, Klebsiella pneumoniae, and Peptostreptococcus species; however, ertapenem lacks activity against P aeruginosa.18,19 FDA-approved indications for ertapenem include community-acquired pneumonia and complicated intra-abdominal, urinary tract, and skin and skin structure infections. Ertapenem, 1 g intravenous daily, is approved for cSSTI due to S aureus (MSSA), S pyogenes, E coli, and Peptostreptococcus species. A study comparing ertapenem and piperacillin-tazobactam for the treatment of mixed anaerobic cSSTIs, intra-abdominal infections, and acute pelvic infections found similar bacterial eradication rates in the two groups. Researchers concluded that ertapenem is as efficacious for the treatment of mixed anaerobic and aerobic cSSTIs.20
The most common adverse events seen with ertapenem include nausea, diarrhea, infusion-related reactions, and headache.18-20 Ertapenem's longer elimination half-life makes once-daily dosing possible--an advantage compared to the other carbapenems. Ertapenem is less likely to cause seizures, making it advantageous over imipenem if Paeruginosais not suspected. Unlike linezolid and daptomycin, ertapenem has a broad spectrum of activity including gram-positive and gram-negative bacteria. If a polymicrobial infection is suspected or confirmed, ertapenem is an appropriate alternative treatment for cSSTI.
Daptomycin: Daptomycin, a cyclic lipopeptide, exhibits rapid, concentration-dependent bactericidal activity against gram-positive bacteria. The exact mechanism of action is not fully understood, but it is thought to insert its lipophilic tail into the cytoplasmic membrane, causing a rapid depolarization and efflux of potassium ions. Bacterial cell death results from a stop in DNA, RNA, and protein synthesis instead of cell lysis.21,22 FDA-approved indications for daptomycin include cSSTIs and S aureus bloodstream infections.
Daptomycin, 4 mg/kg intravenous daily, is approved for the treatment of cSSTI caused by susceptible strains of S aureus (including MRSA), S pyogenes, S agalactiae, Streptococcus dysgalactiae, and vancomycin-susceptible strains of E faecalis.21,23
Two multicenter, randomized, controlled, evaluator-blinded, multinational studies evaluated the efficacy and tolerability of daptomycin versus standard therapy for treatment of patients with cSSTI requiring hospitalization. Daptomycin was found to be as efficacious as standard therapy, with patients receiving daptomycin having a shorter duration of therapy. Of note, only approximately 10% of infections were due to MRSA in this study.24
The most common adverse events seen with daptomycin include constipation, nausea, injection site reactions, and headache. Another less common but important adverse event is elevated levels of creatine phosphokinase (CPK), leading to myopathy. This has been a reason for discontinuation of therapy, and it resolves once therapy is stopped. Patient CPK levels should be monitored weekly, and they should be watched closely for myopathy.21,24 Daptomycin's long half-life and prolonged postantibiotic have the advantage of once-daily dosing. In patients with gram-positive cSSTI that cannot receive vancomycin or linezolid, daptomycin would be an appropriate choice.
Tigecycline: Tigecycline, a structural analogue of minocycline, represents the first glycylcycline antimicrobial to be approved for use in the U.S. Like other tetracyclines, tigecycline inhibits protein synthesis by binding to the 30S ribosomal subunit; however, although the exact mechanism is not yet known, tigecycline is unique because it can overcome or avoid tetracycline resistance. Many organisms that in the past had acquired resistance to tetracycline are now susceptible, extending the spectrum of activity for tigecycline to include many clinically important bacteria such as Streptococcus species, MSSA and MRSA, Enterococcus species, Enterobacteriaceae, and anaerobic bacteria.25,26 Tigecycline has been evaluated in several randomized, double-blind studies evaluating the efficacy and tolerability of tigecycline versus vancomycin and aztreonam for treatment of patients with cSSTI requiring hospitalization. Tigecycline was found to be as efficacious as standard therapy when clinical cure rates and microbiological data were compared.27-29
Tigecycline is generally well tolerated, with mild to moderate nausea and vomiting being the most commonly encountered adverse effects. After an initial loading dose of 100 mg, tigecycline can be administered intravenously as a 50-mg dose twice daily.25,26 Although more studies are needed against other commonly used comparator regimens, tigecycline has shown promise as an alternative for the initial empiric treatment of cSSTIs.
Investigational Agents: A novel class of antibiotics, semisynthetic lipoglycopeptides, consisting of dalbavancin and telavancin, is currently under investigation for the treatment of cSSTI. Two-dose dalbavancin and daily telavancin have been found to be efficacious in the treatment of gram-positive cSSTIs when compared to standard therapy.30-33 An advantage of dalavancin is its long elimination half-life (nine–12 days) allowing for once-weekly dosing. Further studies are need with both agents before they are approved by the FDA.
Ceftobiprole is a novel bactericidal cephalosporin with a broad spectrum of activity against gram-positive and gram-negative bacteria, including MRSA, vancomycin intermediate S aureus (VISA), and vancomycin-resistant S aureus (VRSA). Unlike the other cephalosporins, ceftobiprole inhibits all transpeptidases, including PBP 2a, the enzyme responsible for beta-lactam drug resistance in MRSA. Phase III trials are currently under way, and this drug has been placed on the fast track for FDA approval.
The Pharmacist's Role/Conclusion
In the past several years, the epidemiology of SSTIs has changed. The
incidences of CA-MRSA and macrolide-resistant streptococci are increasing,
which has created difficult clinical scenarios. The changing nature of cSSTIs
presents new challenges for patient care in which effective drug therapy is
necessary, and a number of new antibiotic therapies are available or in
development. As professionals versed in drug therapy, pharmacists are
positioned to contribute to effective patient care by providing
recommendations regarding effective medication therapy for all severities of
SSTIs. Standard therapy agents along with adjunctive therapy (i.e., incision
and drainage) should continue as first-line therapy for cSSTI. This leaves the
newer agents for the more difficult or hard-to-treat cases. Linezolid is a
good drug choice if a patient can be discharged and oral therapy is
appropriate. Ertapenem is an option for patients with polymicrobial
infections, and daptomycin is an alternative to vancomycin or linezolid in
treating gram-positive infections. Tigecycline would be an appropriate
alternative to any of the above mentioned agents for empiric therapy.
References
1. Nichols RL, Florman S. Clinical presentations of soft-tissue infections and
surgical site infections. Clin Infect Dis. 2001;33(suppl 2):S84-S93.
2. Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the
diagnosis and management of skin and soft-tissue infections. Clin Infect Dis
. 2005;41:1373-1406.
3. DiNubile MJ, Lipsky BA. Complicated infections of skin and skin structures:
when the infection is more than skin deep. J Antimicrobial Chemotherapy
. 2004;53(suppl)2:ii37-2:ii50.
4. Tice AD, Poretz D, Cook F, et al. Medicare coverage of outpatient
ambulatory intravenous antibiotic therapy: a program that pays for itself.
Clin Infect Dis. 1998;27:1415-1421.
5. Bowler PG, Duerden BI, Armstrong DG. Wound microbiology and associated
approaches to wound management. Clinical Microbiology Reviews.
2001;14:244-269.
6. Eron LJ, Lipsky BA, Low DE, et al. Managing skin and soft tissue
infections: expert panel recommendations on key decision points. J
Antimicrobial Chemotherapy. 2003;52(suppl)1:i3-1:i17.
7. Uncomplicated and complicated skin and skin structure infections--developing
antimicrobial drugs for treatment. Guidance for Industry: Center for Drug
Evaluation and Research (CDER).1998. Available at:
http://www.fda.gov/cder/guidance/2566dft.pdf.
8. Majeski JA, John JF Jr. Necrotizing soft tissue infections: a guide to
early diagnosis and initial therapy. Southern Medical J.
2003;96:900-905.
9. King MD, Humphrey BJ, Wang YF. Emergence of community-acquired
methicillin-resistant Staphylococcus aureus USA 300 clone as the predominant
cause of skin and soft-tissue infections. Annals of Internal Med.
2006;144:309-317.
10. Jallali N, Withey S, Butler PE. Hyperbaric oxygen as adjuvant therapy in
the management of necrotizing fasciitis. American J Surgery.
2005;189:462-466.
11. Nichols RL, Graham DR, Barriere SL, et al. Treatment of hospitalized
patients with complicated gram-positive skin and skin structure infections:
two randomized, multicentre studies of quinupristin/dalfopristin versus
cefazolin, oxacillin or vancomycin. Synercid Skin and Skin Structure Infection
Group. J Antimicrobial Chemotherapy. 1999;44:263-273.
12. Delgado G Jr, Neuhauser MM, Bearden DT. Quinupristin-dalfopristin: an
overview. Pharmacotherapy. 2000;20:1469-1485.
13. Moellering RC. Linezolid: the first oxazolidinone antimicrobial. Anna
Intern Med. 2003;138:135-142.
14. Sharpe JN, Shively EH, Polk HC, Jr. Clinical and economic outcomes of oral
linezolid versus intravenous vancomycin in the treatment of MRSA-complicated,
lower-extremity skin and soft-tissue infections caused by
methicillin-resistant Staphylococcus aureus. American J Surgery.
2005;189:425-428.
15. Weigelt J, Itani K, Stevens D, et al. Linezolid versus vancomycin in
treatment of complicated skin and soft tissue infections. Antimicrobial
Agents and Chemotherapy. 2005;49:2260-226.
16. Donowitz GR. Oxazolidinones. In: Mandell GL, Douglas RG, Bennett JE, Dolin
R, eds. Mandell, Dougals, and Bennett's Principles and Practice of
Infectious Diseases. 6th ed. New York, NY: Elsevier/Churchill Livingstone;
2005:436-440.
17. Stevens DL, Smith LG, Bruss JB, et al. Randomized comparison of linezolid
(PNU-100766) versus oxacillin-dicloxacillin for treatment of complicated skin
and soft tissue infections. Antimicrobial Agents and Chemotherapy.
2000;44:3408-3413.
18. Keating GM, Perry CM. Ertapenem: a review of its use in the treatment of
bacterial infections. Drugs. 2005;65:2151-2178.
19. Chambers HF. Other B-lactam antibiotics. In: Mandell GL, Douglas RG,
Bennett JE, Dolin R, eds. Mandell, Douglas, and Bennett's Principles and
Practice of Infectious Diseases. 6th ed. New York: Elsevier/Churchill
Livingstone; 2005:311-318.
20. Tellado J, Woods GL, Gesser R, et al. Ertapenem versus
piperacillin-tazobactam for treatment of mixed anaerobic complicated
intra-abdominal, complicated skin and skin structure, and acute pelvic
infections. Surgical Infections. 2002;3:303-314.
21. Carpenter CF, Chambers HF. Daptomycin: another novel agent for treating
infections due to drug-resistant gram-positive pathogens. Clin Infect Dis
. 2004;38:994-1000.
22. Steenbergen JN, Alder J, Thorne GM, Tally FP. Daptomycin: a lipopeptide
antibiotic for the treatment of serious Gram-positive infections. J
Antimicrobial Chemotherapy. 2005;55:283-238.
23. Murray BE, Nannini EC. Glycopeptides (vancomycin, teicoplanin),
streptogramins (quinupristin-dalfopristin), and lipopeptides (daptomycin). In:
Mandell GL, Douglas RG, Bennett JE, Dolin R, eds. Mandell, Dougals, and
Bennett's Principles and Practice of Infectious Diseases. 6th ed.
New York, NY: Elsevier/Churchill Livingstone; 2005:417-434.
24. Arbeit RD, Maki D, Tally FP, et al. The safety and efficacy of daptomycin
for the treatment of complicated skin and skin-structure infections. Clin
Infect Dis. 2004;38:1673-1681.
25. Zhanel GG, Karlowsky JA, Rubinstein E, Hoban DJ. Tigecycline: a novel
glycylcycline antibiotic. Expert Review of Anti-infective Therapy.
2006;4:9-25.
26. Doan TL, Fung HB, Mehta D, Riska PF. Tigecycline: a glycylcycline
antimicrobial agent. Clinical Therapeutics. 2006;28:1079-1106.
27. Breedt J, Teras J, Gardovskis J, et al. Safety and efficacy of tigecycline
in treatment of skin and skin structure infections: results of a double-blind
phase 3 comparison study with vancomycin-aztreonam. Antimicrobial Agents
and Chemotherapy. 2005;49:4658-4566.
28. Ellis-Grosse EJ, Babinchak T, Dartois N, et al. The efficacy and safety of
tigecycline in the treatment of skin and skin-structure infections: results of
2 double-blind phase 3 comparison studies with vancomycin-aztreonam. Clin
Infect Dis. 2005;41(suppl 5):S341-S353.
29. Sacchidanand S, Penn RL, Embil JM, et al. Efficacy and safety of
tigecycline monotherapy compared with vancomycin plus aztreonam in patients
with complicated skin and skin structure infections: results from a phase 3,
randomized, double-blind trial. Int J Infect Dis. 2005;9:251-261.
30. Jauregui LE, Babazadeh S, Seltzer E, et al. Randomized, double-blind
comparison of once-weekly dalbavancin versus twice-daily linezolid therapy for
the treatment of complicated skin and skin structure infections. Clin
Infect Dis. 2005;41:1407-1415.
31. Seltzer E, Dorr MB, Goldstein BP, Perry M, et al. Once-weekly dalbavancin
versus standard-of-care antimicrobial regimens for treatment of skin and
soft-tissue infections. Clin Infect Dis. 2003;37:1298-1303.
32. Stryjewski ME, O'Riordan WD, Lau WK, et al. Telavancin versus standard
therapy for treatment of complicated skin and soft-tissue infections due to
gram-positive bacteria. Clin Infect Dis. 2005;40:1601-1607.
33. Stryjewski ME, Chu VH, O'Riordan WD, et al. Telavancin versus standard
therapy for treatment of complicated skin and skin structure infections caused
by gram-positive bacteria: FAST 2 study. Antimicrobial Agents and
Chemotherapy. 2006;50:862-867.
To comment on this article, contact editor@uspharmacist.com.





