US Pharm. 2011;36(1):44-55.
Narcolepsy is an incurable neurologic sleep disorder characterized by an abnormality in onset and offset of rapid eye movement (REM) and non-REM (NREM) sleep.1 Four main symptoms are associated with narcolepsy: cataplexy, excessive daytime sleepiness (EDS), sleep paralysis, and hypnagogic hallucinations.2 Experiencing all four symptoms is not required for a narcolepsy diagnosis.
Narcoleptic patients often complain of sleep deprivation, constant fatigue, insomnia, and fragmented nighttime sleep. Disproportionate amounts of sleepiness are also experienced during the day and can last from seconds to minutes. These episodes are known as EDS. Involuntary, sudden lapses into unconsciousness are called sleep attacks.
Cataplexy, a symptom exclusive to narcolepsy, is a strong loss of bilateral muscle tone that can last from a few seconds up to 30 minutes. It is stimulated by emotions, such as laughter, anger, or surprise.1 Another symptom of narcolepsy is sleep paralysis. This is a brief episode involving the loss of voluntary muscle tone that usually happens immediately upon awakening or going to sleep. It is often a frightening experience, where patients cannot move their body or speak, although they are conscious of their surroundings.2,3 The patient’s consciousness during the episode differentiates sleep paralysis from sleep attacks.
Some patients may experience hypnagogic and hypnopompic hallucinations. Hypnagogic hallucinations typically occur while falling asleep. They involve abnormal visual and vivid auditory hallucinations. Hypnopompic hallucinations are similar, but happen upon awakening.3
Individuals with narcolepsy are often obese, with a higher than normal body mass index (BMI), probably due to low metabolic rate.4
Epidemiology
The onset of narcolepsy typically begins in the teenage years and young adulthood, and then persists for life.5 It is moderately more predominant in men than in women.6,7
The prevalence of narcolepsy with cataplexy is estimated to be 25 to 50 per 100,000 people, while narcolepsy without cataplexy is estimated higher (56 per 100,000 people).6 The incidence of narcolepsy with cataplexy is estimated at 0.74 per 100,000 person-years, while narcolepsy with or without cataplexy is at 1.37 per 100,000 person-years.6
Ohayon and Okun found that narcolepsy possibly has a genetic component, since the risks for narcolepsy with cataplexy are 2.8% to 5.6% in first-degree relatives.5 An additional 1.7% to 5% of relatives had narcolepsy without cataplexy.
Etiology
Several studies have shown a high predominance of the human leukocyte antigen (HLA)-DR2, specifically HLA-DQB1*0602, which is found in 95% of narcoleptics with cataplexy and in 41% of narcoleptics without cataplexy, indicating narcolepsy may be an autoimmune disease.3,8 Carrying HLA-DQB1*0602 indicates that individuals are predisposed to narcolepsy, but this factor is not enough on its own to diagnose someone with the disease, since it is found in about 12% to 38% of nonnarcoleptics.2,9
The discovery of hypocretin-1 and -2, also known as orexin A and B, respectively, gave rise to the hypothesis that narcolepsy is caused by a selective early hypocretin neuron loss occurring within and around the lateral area in the hypothalamus; however, the exact cause of the neuronal loss in narcolepsy is still unknown.10,11 Orexin was identified as the major sleep-modulating neurotransmitter, responsible not only for sleep but also for appetite and metabolism. Most narcoleptic patients without cataplexy have normal cerebral spinal fluid (CSF) concentrations of orexin A, whereas >90% of narcoleptic subjects with cataplexy have undetectable orexin A in their CSF.11
In a recent study by Cvetkovic-Lopes et al, it was discovered that transcript encoding Tribbles homolog 2 (Trib2) is an autoantigen in narcolepsy.12 Trib2-specific antibodies destroy hyprocretin neurons, which eventually result in hyprocretin deficiency.12
Diagnosis
The diagnostic criteria show that there are two kinds of narcolepsy—narcolepsy with cataplexy and narcolepsy without cataplexy. Narcolepsy with cataplexy is defined as cataplexy along with recurrent daytime naps or lapses into sleep that occur almost daily for at least 3 months.13 Narcolepsy without cataplexy consists of a patient complaint of excessive sleepiness or sudden muscle weakness, along with associated features, including sleep paralysis, hypnagogic hallucinations, automatic behaviors, or disrupted major sleep episodes.13 Of course, narcolepsy will not be diagnosed if any other medical or mental disorders account for the symptoms.13
A polysomnogram (PSG) and a multiple sleep latency test (MSLT) can assist in the diagnosis of narcolepsy without cataplexy (TABLE 1).13-16 Both are used to measure the excessive sleepiness and sleep-onset REM periods. A PSG and an MSLT, along with the above-mentioned symptoms, are necessary to affirm minimum diagnosis.13
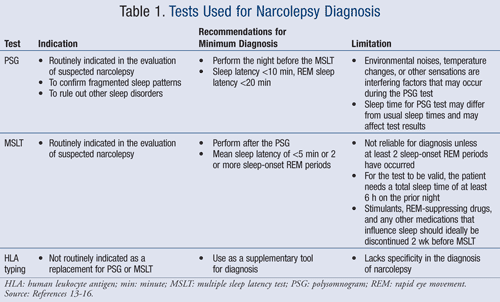
HLA typing is an assisting method that some clinicians find helpful to make the diagnosis of narcolepsy. It shows the presence of HLA-DR2 or HLA-DQB1*0602 in most patients with narcolepsy. While this indicates whether the individual has a genetic predisposition to the disease, it is not routinely used as a replacement for the polysomnogram and MSLT.13,14
Since the discovery of CSF hypocretin-1, it is possible to have hypocretin-1 levels measured by performing a lumbar puncture. Detecting levels of less than 110 ng/L may indicate that the patient has narcolepsy.17
Nonpharmacologic Treatment
Encouraging patients to inform their family, coworkers, or faculty at school is important to avoid the misperception of laziness or incompetence. Regular naps and good sleep hygiene should be part of a narcoleptic patient’s lifestyle. Support groups can also be recommended for patients to learn coping mechanisms. Patients should be counseled to avoid alcohol and other CNS-depressant drugs that may negatively impact wakefulness.
Pharmacologic Treatment
The goal of the following pharmacologic treatments is to reduce the symptoms of narcolepsy and improve quality of life. Once started on these medications (TABLE 2), patients should be seen every time a medication titration is needed and every 6 to 12 months to evaluate any improvements and adverse effects.1
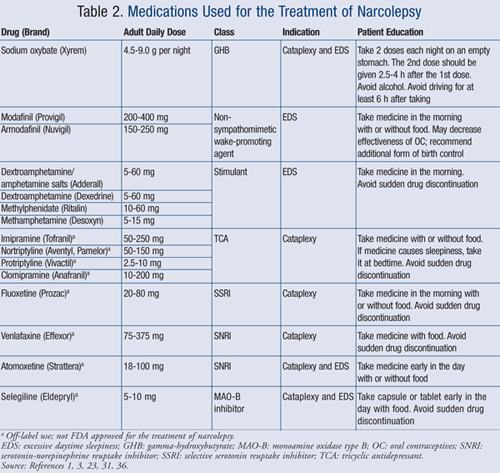
Sodium Oxybate (Xyrem, gamma-hydroxybutyrate [GHB]): In 2002, sodium oxybate was approved by the FDA for treatment of narcolepsy with cataplexy. The medication has been shown to prolong slow-wave sleep, improve REM sleep, and decrease fragmented sleep during the night. Sodium oxybate improves symptoms of EDS, cataplexy, and disrupted sleep due to narcolepsy. The drug may also be effective for sleep paralysis and hypnagogic hallucinations.1,18,19
GHB is a metabolite of gamma-aminobutyric acid (GABA) that works as a partial agonist at GABA-B receptors and an agonist at GHB receptors. This action may contribute to promoting slow-wave sleep and decreasing cataplexy.18
GHB may improve cataplexy symptoms faster than EDS symptoms. Stimulants or other alerting drugs can be taken temporarily until the medication reaches its full effects, which can take up to 3 months.3 Dosage should be gradually increased over time. It is rewarding for patients to know that after 3 months of starting treatment, they may need only sodium oxybate to control all narcolepsy symptoms.3 GHB is supplied in a liquid form to be given at bedtime 4.5 to 9 g; due to its short half-life, a second dose is necessary 2.5 to 4 hours later.19
Adverse effects include nausea, headache, dizziness, confusion, and incontinence. The medication should not be administered concomitantly with alcohol or any other CNS depressant due to risk of respiratory depression.19 A clinically moderate dose to treat narcolepsy does not cause withdrawal symptoms or tolerance.20 It can be safely administered with stimulants or modafinil.
In a randomized, multicenter, double-blind, placebo-controlled trial of 285 patients with narcolepsy, sodium oxybate was shown to be effective and safe, and it improved daily function, sleep quality, continuity, and overall quality of life.21,22
A disadvantage of this medication is that it has a significant abuse potential. To obtain the medication, the patient and the physician must be enrolled in the Xyrem Success Program.19 Sodium oxybate comes from a central pharmacy. The pharmacist calls the patient once he or she has received the medication to answer any questions or concerns, and continuously monitors for any future diversion or drug misuse.
Modafinil (Provigil): Modafinil is the first-line therapy for EDS. Modafinil has limited effectiveness on cataplexy, and coadministration of two or more classes of drugs may be needed in select patients to target symptoms of cataplexy and EDS.23,24
The mechanism of action of modafinil is not fully understood. Recent studies suggest modafinil may act via stimulation of hypocretin-containing neurons in the hypothalamus or through inhibition of dopamine reuptake.18,25 Modafinil can increase levels of dopamine in the nucleus accumbens and may have the potential for abuse since many other drugs of abuse work through the same mechanism (e.g., cocaine, methylphenidate).26 Modafinil is currently a schedule IV controlled substance, and reinforcing effects have been demonstrated in animal studies; however, it lacks the same reinforcing effects that cocaine and methylphenidate demonstrate.27,28 Postmarketing analysis suggests modafinil has limited potential for large-scale abuse; nevertheless, patients should be monitored for signs of abuse and drug-seeking behavior.29 The dose administered for the treatment of narcolepsy is 200 and 400 mg/day.30,31
Modafinil is well tolerated and has minimal side effects; however, headache, nausea, nervousness, anxiety, and insomnia may occur. Modafinil has a longer elimination half-life of approximately 15 hours compared to traditional stimulants.31 Patients on medications metabolized through CYP2C19 should be monitored when using modafinil since it has CYP2C19 inhibitory properties. Modafinil can also induce CYP3A4, possibly reducing the effectiveness of oral contraceptives and other medications commonly metabolized by CYP3A4. For that reason, it is important to counsel women to use other methods of contraception while taking this medication. In patients with severe hepatic impairment, a half dose of modafinil is recommended. Elevation in blood pressure may also occur; therefore, patients suffering from hypertension should have their blood pressure monitored.31 Modafinil should be used cautiously in patients with a history of heart attack or unstable angina.
A randomized trial showed that modafinil 400 mg/day improved overall energy, productivity, attention, and self-esteem in patients with narcolepsy compared to placebo.30 Black and Houghton conducted a study of 270 narcoleptic patients and found that when sodium oxybate and modafinil are administered together, patients’ EDS symptoms improve more than with either agent alone.24 Both medications given concomitantly seem to be well tolerated, although there may be a higher incidence for side effects.24
Overall, modafinil is an effective treatment option for narcolepsy since it promotes wakefulness without causing rebound EDS, and it can also be used with other medications such as GHB to treat cataplexy symptoms.24
Armodafinil (Nuvigil): In 2007, the FDA approved armodafinil, the R-enantiomer of modafinil, which improves wakefulness in EDS. Armodafinil is an option for patients who, after trying once-daily modafinil, still experience sleepiness later in the day and probably require multiple doses.32 The recommended dosage for armodafinil is 150- or 250-mg once daily in the morning.31
Armodafinil binds to the dopamine transporter and inhibits dopamine reuptake, which may result in an increase of extracellular dopamine levels in the brain. Effects on the dopamine transporter are the most likely mechanism for armodafinil’s wake-promoting effects; however, the exact mechanism of action is unknown.18
In a multicenter, randomized, placebo-controlled, double-blind study, 196 narcoleptic patients were enrolled and received 150- or 250-mg/day or placebo. A statistically significant improvement was seen in wakefulness throughout the day on the Maintenance of Wakefulness Test (MWT) at each dose compared to placebo.31
Armodafinil has a side-effect profile similar to modafinil’s and overall is very similar to modafinil, except that armodafinil produces higher plasma concentrations later in the day.31
Amphetamines and Methylphenidate: CNS stimulants such as dextroamphetamine/amphetamine salts, dextroamphetamine, and methylphenidate are FDA-approved agents for the treatment of narcolepsy. These medications are effective for EDS. Clinical judgment should be utilized accordingly when prescribing psychostimulants, since these agents are not recommended as a first option due to high abuse potential, tolerance, and a significant side-effect profile.3,23,27
Other Treatment Options: Cataplexy may also be improved with low doses of antidepressants such as tricyclic antidepressants (TCAs, e.g., imipramine, nortriptyline, protriptyline, clomipramine), fluoxetine, venlafaxine, and atomoxetine.23,33,34 These medications are not FDA approved for the treatment of narcolepsy; however, clinical experience indicates they may be effective for the treatment of cataplexy, sleep paralysis, and hypnagogic hallucinations.23,34 The disadvantage with TCAs is rebound cataplexy, which may be experienced if the medication is discontinued abruptly.3
Selegiline is another antidepressant that has been used off-label for treatment of cataplexy and EDS in narcoleptic patients. The mechanism of action is through REM suppression and an increase in REM latency.1,23
Discretion should be used when using off-label treatments for narcolepsy, since there is a lack of concrete evidence compared to FDA-approved treatments.
Future Therapies: Intranasal hypocretin-1 replacement is a therapy being developed that crosses the blood–brain barrier and appears to have a great potential for the treatment of narcolepsy.30 Animal studies have shown thioperamide, a histamine (H3) antagonist, may improve wakefulness by blocking histamine autoreceptors. This therapy is currently being researched and aims to improve EDS in patients with narcolepsy.35 These investigational agents may hold some promise for the future, although further research is needed.
Conclusion
Narcolepsy may affect patients in several areas of their lives, including occupationally, with social functioning, with daily activities, and during leisure times. Much has been researched and discovered regarding narcolepsy over the years, although a complete understanding of the cause of the disease is still unknown. Modafinil and sodium oxybate are the best-recognized treatments available for patients with narcolepsy. Ongoing research is being performed to find more optimal treatments. Future studies should focus on identifying other factors that could contribute to hypocretin neuronal loss.
Pharmacists should be informed on the typical symptoms of narcolepsy, such as EDS, cataplexy, hallucinations, and sleep paralysis. If these symptoms are recognized, patients should be referred to their primary care physician or a sleep specialist. Pharmacists should also be aware of the counseling points regarding the most common medications used to treat narcolepsy, as well as their main side effects (TABLE 2).
REFERENCES
1. Dopp JM, Phillips BG. Sleep disorders. In: DiPiro JT, Talbert RL, Yee GC, et al, eds. Pharmacotherapy: A Pathophysiologic Approach. 7th ed. New York, NY: McGraw-Hill; 2008:1191-1201.
2. Mignot E. Genetics of narcolepsy and other sleep disorders. Am J Hum Genet. 1997;60:1289-1302.
3. Cao M. Advances in narcolepsy. Med Clin N Am. 2010;94:541-555.
4. Dahmen N, Bierbrauer J, Kasten M. Increased prevalence of obesity in narcoleptic patients and relatives. Eur Arch Psychiatry Clin Neurosci. 2001;251:85-89.
5. Ohayon MM, Okun ML. Occurrence of sleep disorders in the families of narcoleptic patients. Neurology. 2006;67:703-705.
6. Longstreth WT Jr, Koepsell TD, Ton TG, et al. The epidemiology of narcolepsy. Sleep. 2007;30:13-26.
7. Silber MH, Krahn LE, Olson EJ, Pankratz VS. The epidemiology of narcolepsy in Olmsted County, Minnesota: a population based study. Sleep. 2002;25:197-202.
8. Nishino S, Ripley B, Overeem S, et al. Low cerebrospinal fluid hypocretin (orexin) and altered energy homeostasis in human narcolepsy. Ann Neurol. 2001;50:381-388.
9. Rogers AE, Meehan J, Guilleminault C, et al. HLA DR15(DR2) and DQB1*0602 typing studies in 188 narcoleptic patients with cataplexy. Neurology. 1997;48:1550-1556.
10. Sakurai T, Amemiya A, Ishii M, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573-585.
11. Lodi R, Tonon C, Vignatelli L, et al. In vivo evidence of neuronal loss in the hypothalamus of narcoleptic patients. Neurology. 2004;63:1513-1515.
12. Cvetkovic-Lopes V, Bayer L, Dorsaz S, et al. Elevated Tribbles homolog 2–specific antibody levels in narcolepsy patients. J Clin Invest. 2010;120:713-719.
13. American Academy of Sleep Medicine. International Classification of Sleep Disorders, Revised: Diagnostic and Coding Manual. Chicago, IL: American Academy of Sleep Medicine; 2001:38-43.
14. Kushida CA, Littner MR, Morgenthaler T, et al. Practice parameters for the indications for polysomnography and related procedures: an update for 2005. Sleep. 2005;28:499-521.
15. Standards of Practice Committee of the American Academy of Sleep Medicine. Practice parameters for clinical use of the multiple sleep latency test and the maintenance of wakefulness test. Sleep. 2005;28:113-121.
16. Pagana KD, Pagana TJ. Mosby’s Manual of Diagnostic and Laboratory Tests. 4th ed. St. Louis, MO: Elsevier Health Sciences; 2009.
17. Mignot E, Lammers GJ, Ripley B, et al. The role of cerebrospinal fluid hypocretin measurement in the diagnosis of narcolepsy and other hypersomnias. Arch Neurol. 2002;59:1553-1562.
18. Stahl SM. Stahl’s Essential Psychopharmacology. 3rd ed. New York, NY: Cambridge University Press; 2008.
19. Xyrem (sodium oxybate) package insert. Palo Alto, CA: Jazz Pharmaceuticals, Inc; November 2005.
20. US Xyrem Multi-Center Study Group. The abrupt cessation of therapeutically administered sodium oxybate (GHB) does not cause withdrawal symptoms. J Toxicol Clin Toxicol. 2003;41:131-135.
21. Weaver TE, Cuellar N. A randomized trial evaluating the effectiveness of sodium oxybate therapy on quality of life in narcolepsy. Sleep. 2006;29:1189-1194.
22. The U.S. Xyrem Multicenter Study Group. A randomized, double-blind, placebo-controlled multicenter trial comparing the effects of three doses of orally administered sodium oxybate with placebo for the treatment of narcolepsy. Sleep. 2002;25:42-49.
23. Morgenthaler TI, Kapur VK, Brown T, et al. Practice parameters for the treatment of narcolepsy and other hypersomnias of central origin. Sleep. 2007;30:1705-1711.
24. Black J, Houghton WC. Sodium oxybate improves excessive daytime sleepiness in narcolepsy. Sleep. 2006;29:939-946.
25. Chemelli RM, Willie JT, Sinton CM, et al. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98:437-451.
26. Volkow ND, Fowler JS, Logan J, et al. Effects of modafinil on dopamine and dopamine transporters in the male human brain: clinical implications. JAMA. 2009;301:1148-1154.
27. Jasinski DR. An evaluation of the abuse potential of modafinil using methylphenidate as a reference. J Psychopharmacol. 2000;14:53-60.
28. Vosburg SK, Hart CL, Haney M, et al. Modafinil does not serve as a reinforcer in cocaine abusers. Drug Alcohol Depend. 2010;106:233-236.
29. Myrick H, Malcolm R, Taylor B, LaRowe S. Modafinil: preclinical, clinical, and post-marketing surveillance—a review of abuse liability issues. Ann Clin Psychiatry. 2004;16:101-109.
30. Beusterien KM, Rogers AE, Walsleben JA, et al. Health-related quality of life effects of modafinil for treatment of narcolepsy. Sleep. 1999;22:757-765.
31. Lexi-Comp Online. Hudson, OH: Lexi-Comp, Inc; 2010. www.lexi.com. Accessed August 10, 2010.
32. Harsh JR, Hayduk R, Rosenberg R, et al. The efficacy and safety of armodafinil as treatment for adults with excessive sleepiness associated with narcolepsy. Curr Med Res Opin. 2006;22:761-774.
33. Mignot E, Nishino S. Emerging therapies in narcolepsy-cataplexy. Sleep. 2005;28:754-763.
34. Rosenthal MS. Physiology and neurochemistry of sleep. Am J Pharm Educ. 1998;62:204-208.
35. Barbier AJ, Berridge C, Dugovic AD, et al. Acute wake-promoting actions of JNJ-5207852, a novel, diamine-based H3 antagonist. Br J Pharmacol. 2004;143:649-661.
36. Wise MS, Arand DL, Auger RR, et al. Treatment of narcolepsy and other hypersomnias of central origin. Sleep. 2007;30:1712-1727.
To comment on this article, contact rdavidson@uspharmacist.com.





