US Pharm. 2018;43(8):21-25.
ABSTRACT: Not only are men less likely than women to see healthcare providers, but they are also more likely to die from pneumonia and influenza-related illnesses. Knowledge of these facts provides pharmacists with a unique opportunity to assist in the prevention and treatment of community-acquired pneumonia, especially in men. Pharmacists can improve adult vaccination rates through the implementation of pneumococcal vaccination programs. In addition, they can help decrease infection relapse rates by counseling patients on the proper use of antibiotics. Pharmacists should work to improve vaccination rates in both men and women, but particularly in men given their less frequent use of healthcare services.
It is well documented that men are less likely than women to use healthcare services.1-3 In a 2013 survey, 68% of men reported seeing a healthcare provider when sick, compared with 83% of women.1 In terms of life expectancy, data from the 2015 National Vital Statistics Report revealed that women live an average of 4.9 years longer than men.4 Of the leading causes of death in 2015, pneumonia and influenza ranked ninth among males of all ages and ethnic backgrounds.5 Men are estimated to be about 25% more likely than women to die from these diseases.6
Pneumonia is the leading cause of infectious disease–related death in the United States. Of the 53,000 deaths in 2013 that were caused by pneumonia, 25,000 were in men. The mortality rate of pneumonia increases in persons aged 65 years and older, with 85% of deaths occurring in this age group. In 2010, more than one million hospitalizations were attributed to pneumonia. Pneumonia-related care in the U.S. cost $16.2 billion in 2013. Of this total, just over $13 billion was spent on hospitalizations, $1 billion was spent on outpatient care, $900 million was spent on emergency visits, and $250 million was spent on prescription medications.6
Several different terms are used in discussions of pneumonia. Some of these terms have been modified for clarity in the 2016 clinical-practice guidelines of the Infectious Diseases Society of America (IDSA) and American Thoracic Society (ATS) on the management of adults with hospital-acquired and ventilator-associated pneumonia.7 Community-acquired pneumonia (CAP) is contracted within the community; hospital-acquired pneumonia is contracted during a hospital stay that is not associated with mechanical ventilation. Ventilator-associated pneumonia is contracted during a hospital stay that is specifically associated with mechanical ventilation. The IDSA/ATS guidelines recommend the removal of the term healthcare-associated pneumonia based on emerging evidence that many of these patients were not at risk for multidrug-resistant organisms—as was previously believed—thereby negating the need for this term.7
From promoting prevention of CAP through the use of immunization programs to ensuring proper antibiotic therapy, pharmacists are uniquely positioned to assist in the treatment and prevention of CAP.
Common Causes of CAP
CAP is caused by a variety of gram-positive, gram-negative, and atypical pathogens; however, a few pathogens have been identified as the most common causes (TABLE 1). The most frequently isolated pathogen is Streptococcus pneumoniae, followed by Mycoplasma pneumoniae, Haemophilus influenzae, and Chlamydophila pneumoniae. Neisseria meningitidis, Pasteurella multocida, and Streptococcus pyogenes have also been identified as likely pathogens, but they are far less likely to cause CAP.8,9 Recent estimates implicate S pneumoniae as the cause of 19.2 cases per 100,000 population among hospitalized patients.10 There are no standardized or rapid-detection tests for atypical pathogens, and these pathogens are not often identified in clinical practice.8

In a recent study, a viral etiology was found in 18% of nonimmunocompromised patients hospitalized with CAP, and 9% of these patients presented only with a viral pathogen.11 More recently, the emergence of the H1N1 strain of influenza A has impacted hospitalization rates for pneumonia.10 In 2011, mortality rates were highest among patients with CAP caused by Klebsiella spp, Staphylococcus aureus, and Pseudomonas.10
Signs and Symptoms
Common signs and symptoms associated with typical bacterial CAP are fever (in 70% of patients), cough (64%), sputum (16%), headache (7%), dyspnea (65%), chest pain (20%), gastrointestinal symptoms (9%), chills (16%), and general discomfort (22%).8,12 In addition, younger patients may experience high fever (≥102°F), rhinorrhea, malaise, and vomiting.9 Some forms of atypical CAP may involve extrapulmonary signs such as headaches, ear pain, mental confusion, abdominal pain, and diarrhea.13,14
Outpatient Versus Inpatient Care
Most patients diagnosed with CAP can be treated in an outpatient setting. However, in certain situations, the patient must be hospitalized. It is important to understand when hospitalization is necessary because inpatient treatment can cost up to approximately 25 times as much as outpatient treatment.8
CURB-65 (Confusion, Uremia, Respiratory rate, low Blood pressure, and age 65 years and older) and PSI (Pneumonia Severity Index) are two scoring systems used to determine whether to admit a patient with CAP to the hospital (or even ICU) or simply provide outpatient treatment. Ultimately, the clinician’s judgment determines the site of care, but the results of these scoring systems should be strongly considered.15
Outpatient Treatment
Antimicrobial treatment should be initiated as soon as possible after CAP is diagnosed. Because the cost of diagnostic testing can be significant and the procedures are invasive, the cause of infection is usually not pursued in an outpatient setting.15
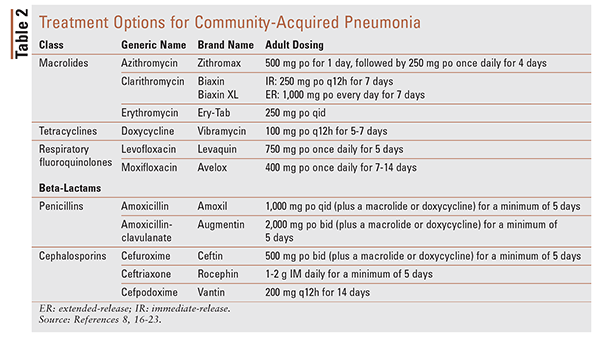
The IDSA/ATS guidelines recommend empirical antibiotic treatment based on various factors, such as the presence of comorbid conditions (e.g., chronic diseases of the heart, lung, liver, or kidneys; diabetes mellitus; alcoholism; and asplenia) and the likelihood of antibiotic resistance. Patients who are considered healthy and have not used antimicrobials within 3 months of CAP diagnosis should be treated with either a macrolide antibiotic (strong recommendation) or doxycycline (weak recommendation). Patients with comorbid conditions should be treated with either a respiratory fluoroquinolone (strong recommendation) or a beta-lactam in conjunction with a macrolide (strong recommendation). Doxycycline may be substituted for the macrolide, if necessary. These recommendations are based not on the specific drug, but rather on the class of antibiotics.8
Guidelines in the United Kingdom and Sweden recommend empiric treatment with either amoxicillin or penicillin as first-line therapy for outpatients showing symptoms of typical bacterial CAP; this is because of the resistance of S pneumoniae to macrolides and doxycycline. For patients showing symptoms of atypical infection, it is suggested that treatment be initiated with either a macrolide or doxycycline, whereas symptomatic treatment is recommended for patients with symptoms of viral pneumonia.15
TABLE 2 lists treatment options (drug, dosing, duration) for CAP.16-23
Prevention via Vaccination
Because CAP is one of the most common infectious diseases associated with increasing antibiotic resistance and leads to substantial morbidity and mortality, one of the Healthy People 2020 initiatives is to increase the percentage of adults who are vaccinated against pneumococcal disease.24 The highest rate of mortality related to pneumococcal disease occurs in adults aged 65 years and older, especially if significant comorbidities exist.25 In 2014, pneumococcal vaccination rates among patients aged 65 years and older were higher in women (61.2%) than in men (55.5%).6 Although pneumococcal vaccines do not prevent all types of CAP, vaccination is considered the safest and most effective method of disease prevention.26 The CDC recommends routine vaccination against pneumococcal disease.
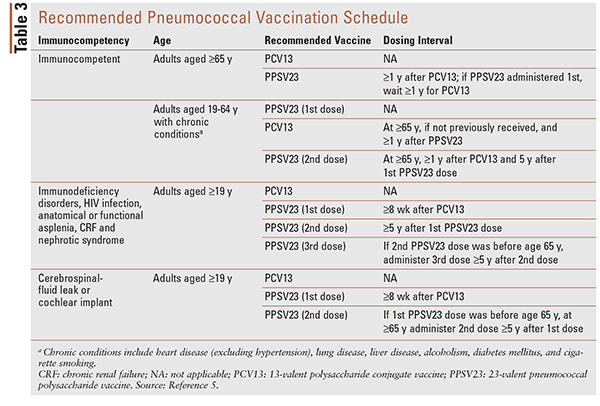
Two pneumococcal vaccines, both of which are administered as 0.5-mL IM injections, are approved in the U.S. The 13-valent polysaccharide conjugate vaccine (PCV13; Prevnar 13) is indicated in children aged 6 weeks through 17 years and adults aged 18 years and older for prevention of pneumococcal disease caused by 13 S pneumoniae strains.27 The 23-valent pneumococcal polysaccharide vaccine (PPSV23; Pneumovax 23) is indicated in adults aged 50 years and older for prevention of pneumococcal disease caused by 23 of the most common S pneumoniae strains (some strains overlap with those contained in the PCV13 vaccine).28
TABLE 3 summarizes the recommended pneumococcal vaccination schedule and dosing intervals as approved by the Advisory Committee on Immunization Practices (ACIP).29,30 When both PCV13 and PPSV23 are to be administered, it is recommended that PCV13 be given first, with a minimum 8-week interval between vaccines in immunocompromised patients and an interval of at least 1 year in immunocompetent patients. This recommendation by the ACIP stems from immunogenicity studies that demonstrated a better response to serotypes common to both vaccines when PCV13 is given first. The longer-interval recommendation for immunocompetent persons is based on the recognition that disease risk is less urgent in that population, the fact that shorter intervals are more likely to result in injection-site reactions, and evidence that suggests that longer intervals may lead to an improved immune response.29 If the PPSV23 dose is inadvertently given less than 1 year after the PCV13 dose in an immunocompetent person, a repeat dose is not necessary.
The Pharmacist’s Role
As noted previously, men are less likely than women to use healthcare services, and they are more likely than women to die from pneumonia and influenza. The Healthy People 2020 objectives regarding vaccination against pneumococcal disease are intended to achieve vaccination rates of 90% for noninstitutionalized adults aged 65 years and older and 60% for high-risk persons aged 18 to 64 years. Based on data from 2008, this represents a needed 30% and 43% increase in vaccination rates, respectively.24 Both male and female patients should also be encouraged to receive an annual influenza vaccination because having influenza increases the risk of developing pneumococcal disease.26
The pharmacist is in an ideal position to help improve adult vaccination rates, particularly in men, through the implementation of pneumococcal vaccination programs. A pneumococcal vaccination history should be obtained by reviewing patient records or by asking patients for their vaccination history. Prescription-profile records may be used to identify patients with chronic disease who are at high risk for pneumococcal disease. Educating patients regarding the benefits of pneumococcal vaccination and administering these vaccines, as permitted by state law, may be easily accomplished in a community-pharmacy setting. Through this screening and vaccination process, the pharmacist can make a significant impact on reducing the incidence of, and complications associated with, pneumococcal disease in both men and women.
Pharmacists can improve infection cure rates in patients diagnosed with CAP by providing thorough patient education on the proper administration of antibiotics and completion of their antibiotic regimen. Preparing patients for expected side effects of antibiotics and how to manage them is vital to adherence. Ensuring that patients understand the importance of completing their antibiotic regimen can help decrease rates of infection relapse, as well as antibiotic resistance. Pharmacists are uniquely positioned to provide this important service to patients, and special efforts should be made to increase vaccination rates in men.
REFERENCES
1. Henry J. Kaiser Family Foundation. Gender differences in health care, status, and use: spotlight on men’s health. www.kff.org/womens-health-policy/fact-sheet/gender-differences-in-health-care-status-and-use-spotlight-on-mens-health. Accessed May 18, 2018.
2. Cleary PD, Mechanic D, Greenley JR. Sex differences in medical care utilization: an empirical investigation. J Health Soc Behav. 1982;23(2):106-119.
3. Hibbard JH, Pope CR. Gender roles, illness orientation and use of medical services. Soc Sci Med. 1983;17(3):129-137.
4. Murphy SL, Xu J, Kochanek KD, et al. Deaths: final data for 2015. Natl Vital Stat Rep. 2017;66(5):1-76.
5. Heron M. Deaths: leading causes for 2015. Natl Vital Stat Rep. 2017;66(6):1-75.
6. American Lung Association. Trends in pneumonia and influenza morbidity and mortality. www.lung.org/assets/documents/research/pi-trend-report.pdf. Accessed May 18, 2018.
7. Kalil AC, Metersky ML, Klompas M, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63(5):e61-e111.
8. Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(suppl 2):S27-S72.
9. Juvén T, Ruuskanen O, Mertsola J. Symptoms and signs of community-acquired pneumonia in children. Scand J Prim Health Care. 2003;21(1):52-56.
10. Wuerth BA, Bonnewell JP, Wiemken TL, Arnold FW. Trends in pneumonia mortality rates and hospitalizations by organism, United States, 2002-2011. Emerg Infect Dis. 2016;22(9):1624-1627.
11. de Roux A, Marcos MA, Garcia E, et al. Viral community-acquired pneumonia in nonimmunocompromised adults. Chest. 2004;125(4):1343-1351.
12. Huijskens EG, Koopmans M, Palmen FM, et al. The value of signs and symptoms in differentiating between bacterial, viral and mixed aetiology in patients with community-acquired pneumonia. J Med Microbiol. 2014;63(Pt 3):441-452.
13. Kishaba T. Community-acquired pneumonia caused by Mycoplasma pneumoniae: how physical and radiological examination contribute to successful diagnosis. Front Med (Lausanne). 2016;3:28.
14. Wang K, Gill P, Perera R, et al. Clinical symptoms and signs for the diagnosis of Mycoplasma pneumoniae in children and adolescents with community-acquired pneumonia. Cochrane Database Syst Rev. 2012;(10):CD009175.
15. Musher DM, Thorner AR. Community-acquired pneumonia. N Engl J Med. 2014;371(17):1619-1628.
16. Zithromax (azithromycin) package insert. New York, NY: Pfizer Labs; January 2013.
17. Biaxin (clarithromycin) package insert. North Chicago, IL: Abbott Laboratories; 2012.
18. Ery-Tab (erythromycin) package insert. Atlanta, GA: Arbor Pharmaceuticals, LLC; July 2013.
19. Vibramycin (doxycycline) package insert. New York, NY: Pfizer Inc; July 2017.
20. Levaquin (levofloxacin) package insert. Raritan, NJ: Ortho-McNeil Pharmaceutical; April 2008.
21. Avelox (moxifloxacin) package insert. Wayne, NJ: Bayer HealthCare Pharmaceuticals Inc; March 2010.
22. Rocephin (ceftriaxone) package insert. South San Francisco, CA: Genentech USA, Inc; 2012.
23. Vantin (cefpodoxime) package insert. New York, NY: Pfizer Inc; 2013.
24. HealthyPeople.gov. Immunization and infectious diseases. www.healthypeople.gov/2020/topics-objectives/topic/immunization-and-infectious-diseases/objectives. Accessed May 24, 2018.
25. CDC. Manual for the surveillance of vaccine-preventable diseases: pneumococcal. www.cdc.gov/vaccines/pubs/surv-manual/chpt11-pneumo.html. Accessed May 24, 2018.
26. CDC. Pneumococcal disease: vaccination. www.cdc.gov/pneumococcal/about/prevention.html. Accessed May 24, 2018.
27. Prevnar 13 (pneumococcal 13-valent conjugate vaccine [diphtheria CRM197 protein]) package insert. Philadelphia, PA: Wyeth Pharmaceuticals Inc; August 2017.
28. Pneumovax 23 (pneumococcal vaccine polyvalent) package insert. Whitehouse Station, NJ: Merck & Co, Inc; May 2015.
29. Kobayashi M, Bennett NM, Gierke R, et al. Intervals between PCV13 and PPSV23 vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2015;64(34):944-947.
30. CDC. Recommended immunization schedule for adults aged 19 years or older, United States, 2018. www.cdc.gov/vaccines/schedules/hcp/imz/adult.html. Accessed May 24, 2018.
To comment on this article, contact rdavidson@uspharmacist.com.





