In the United States, it is estimated that more than 95,000 patients undergo heart valve replacement each year.1 It has become increasingly common to treat hemodynamically significant aortic or mitral valve disease with the implantation of prosthetic cardiac valves, made either from synthetic material, referred to as a mechanical prosthesis, or from biologic tissue, referred to as a bioprosthesis.1 Treatment for valvular disease includes a plan to 1) decrease the risk of further heart damage; 2) manage symptoms with medication, if necessary (TABLE 1 ); 3) repair or replace the valve, if necessary; and 4) communicate to both patient and caregivers the steps for management and self-care, as well as and the importance of follow-up care.
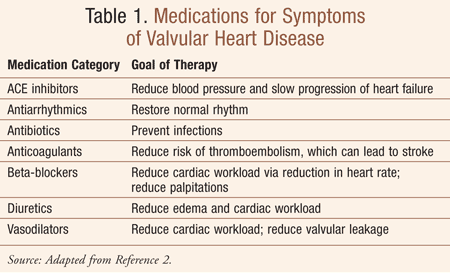
Valve repair and valve replacement are the available choices once the determination has been made that a diseased heart valve needs treatment.2 Valve repair aims to preserve the valve and leaflets and can involve minimal to more extensive surgery. Most often, repair is possible for mitral valve regurgitation and tricuspid valve regurgitation. Valve replacement can involve a less invasive procedure such as transcatheter aortic valve implantation (TAVI) or may require a more involved surgery such as the Ross procedure, where the diseased aortic valve is replaced by the patient’s own pulmonary valve, or the insertion of a new tissue valve or manufactured valve.2 Most valve replacements involve the aortic and mitral valves.2
Indications for Mechanical Heart Valves
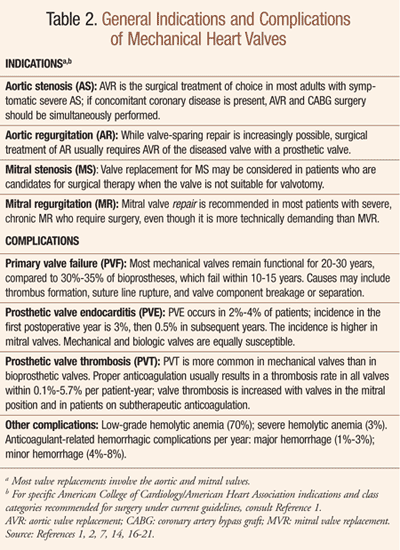
General indications for mechanical heart valves are outlined in TABLE 2 . Of note, valvular aortic stenosis (AS) in older adults is usually caused by stiffening, scarring, and calcification of the aortic valve leaflets; aortic valve replacement (AVR) is the procedure of choice for symptomatic elderly patients with severe AS, including octogenarians.1,3 Causes of acute aortic regurgitation (AR) in older adults may include infective endocarditis, rheumatic fever, aortic dissection, and trauma secondary to prosthetic valve surgery, among others.3 Chronic AR in older adults may be due to valve leaflet disease (secondary to AS, rheumatoid arthritis, ankylosing spondylitis, other conditions) or aortic root disease (associated with systemic hypertension, rheumatoid arthritis, systemic lupus erythematosus).3 Age-associated increased prevalence of AR has been linked to aortic valve thickening.4-6
Complications of Valve Replacement
Complications of mechanical valves are outlined in TABLE 2 . Acute failure of a prosthetic aortic valve causes a rapidly progressive left ventricular volume overload; myocardial ischemia may follow acute AR, even in the absence of coronary artery disease.1 In general, bioprosthetic valves have functional properties (e.g., resistance to thrombosis) more similar to native valves; mechanical valves tend to be thrombogenic and require lifelong anticoagulation.1 Prosthetic valve thrombosis (PVT) and arterial thromboembolism are more common in mechanical valves than in bioprosthetic valves; PVT is increased with valves in the mitral position and in patients on subtherapeutic anticoagulation.1
The type of prosthesis chosen is therefore determined by the patient’s likely longevity and ability to tolerate anticoagulation.7 Proper anticoagulation usually results in a thrombosis rate in all valves within 0.1%-5.7% per patient-year.1 To minimize this risk, oral anticoagulation alone or with the addition of antiplatelet drugs has been used; the effectiveness and safety of the latter strategy is considered an important issue.8 Mechanical valves are not prone to structural valve deterioration, so most remain functional for 20-30 years, compared to 30%-35% of bioprostheses, which fail within 10-15 years.1
Dabigatran
Dabigatran etexilate, the prodrug for the active moiety dabigatran, is a direct thrombin inhibitor currently indicated to reduce the risk of stroke and systemic embolism in patients with nonvalvular atrial fibrillation.9-12 Since thrombin enables the conversion of fibrinogen into fibrin during the coagulation cascade, its inhibition prevents the development of a thrombus; both free and clot-bound thrombin and thrombin-induced platelet aggregation are inhibited by the active moiety.11 After the discovery of warfarin, dabigatran was the first oral anticoagulant to be approved in 50 years.9 Clinicians had hoped that a novel oral direct clotting inhibitor would provide either similar or advanced protection for patients with mechanical heart valves without the warfarin-associated need for strict and frequent monitoring, dietary restrictions, or multiple medication interactions.13
Postmarketing Experience: A randomized clinical trial (RE-ALIGN), however, showed that dabigatran’s side effects were even more problematic than those of warfarin in patients with mechanical heart valves. In this trial, 252 patients who had an aortic or mitral valve replacement were randomly assigned to either dose-adjusted warfarin or dabigatran 150, 220, or 300 mg twice daily.14 The study was terminated early secondary to the occurrence of significantly more thromboembolic events (valve thrombosis, stroke, transient ischemic attack, and myocardial infarction) and excessive major bleeding (predominantly postoperative pericardial effusions requiring intervention for hemodynamic compromise) with dabigatran compared with warfarin. Changes to dabigatran’s prescribing information ensued.14
Black Box Warning: An FDA black box warning was added to the medication label warning against using dabigatran and similar medications in patients with mechanical heart valves as a result of this clinical trial, which was funded by Boehringer Ingelheim, the maker of dabigatran (Pradaxa).10,11,13 Additionally, the following adverse reactions have been identified during postapproval use of dabigatran: angioedema, thrombocytopenia, and esophageal ulcer.11 It is not always possible to reliably estimate the frequency of these reactions or establish a causal relationship to drug exposure since they have been reported voluntarily from a population of uncertain size.11 Pharmacists are encouraged to familiarize themselves with MedWatch: The FDA Safety Information and Adverse Event Reporting Program for clinically important safety information and reporting of serious problems with human medical products.
Risk of Bleeding: Dabigatran increases the risk of bleeding and can cause significant and possibly fatal bleeding. Signs or symptoms of blood loss (e.g., a drop in hemoglobin and/or hematocrit or hypotension) should be promptly evaluated.10,11 This agent should be discontinued in patients with active pathologic bleeding; risk factors for bleeding include concomitant use of medications that increase the risk of bleeding (e.g., antiplatelet agents, heparin, fibrinolytic therapy, chronic use of nonsteroidal anti-inflammatory drugs [NSAIDs]).10,11 Seniors may be at greater risk owing to comorbidities requiring expansive and complex medication regimens that include many of the offending concomitant medications noted above. The elderly are a high-risk population for adverse effects from NSAIDs; up to 60% of elderly people can develop peptic ulceration and/or hemorrhage asymptomatically.15 Renal impairment increases anticoagulant activity and half-life.
There is no reversal agent available for dabigatran. While hemodialysis can remove dabigatran, clinical experience is limited with regard to hemodialysis as a treatment for bleeding.10,11 While their use has not been evaluated, activated prothrombin complex concentrates, recombinant factor VIIa, or concentrates of factors II, IX, or X may be considered; protamine sulfate and vitamin K are not expected to affect dabigatran anticoagulant activity.10,11 The administration of platelet concentrates should be considered where thrombocytopenia is present or long-acting antiplatelet drugs have been used.10,11

Conclusion
Pharmacists are encouraged to keep abreast of postmarketing randomized clinical trials for information to support or challenge premarketing safety and efficacy data for the direct thrombin inhibitor dabigatran, including updated dosing guidelines, drug interactions, and specific monitoring recommendations.
REFERENCES
1. Yang EH, Lin J. Mechanical heart valves. Medscape. http://emedicine.medscape.com/article/1971097-overview#aw2aab6b2. Accessed January 17, 2014.
2. Heart valves are for life. American
Heart Association. Content reviewed 02/18/13.
www.heart.org/HEARTORG/Conditions/More/HeartValveProblemsandDisease/Heart-Valve-Problems-and-Disease_UCM_450280_SubHomePage.jsp.
Accessed January 20, 2014.
3. Aronow WS. Valvular heart disease. In: Fillit HM, Rockwood K, Woodhouse K, eds. Brocklehurst’s Textbook of Geriatric Medicine and Gerontology. 7th ed. Philadelphia, PA: Saunders Elsevier; 2010:312-326.
4. Margonato A, Cianflone D, Carlino M,
et al. Frequency and significance of aortic valve thickening in older
asymptomatic patients and its relation to aortic regurgitation. Am J Cardiol. 1989; 64:1061-1062.
5.
Akasaka T, Yoshikawa J, Yoshida K, et al. Age-related valvular regurgitation: a study by pulsed Doppler echocardiography. Circulation. 1987;76:262-265.
6. Aronow WS, Kronzon I. Correlation of
prevalence and severity of aortic regurgitation detected by pulsed
Doppler echocardiography with the murmur of aortic regurgitation in
elderly patients in a long-term health care facility. Am J Cardiol. 1989; 63:128-129.
7. Rahimtoola SH. Choice of prosthetic heart valve in adults: an update. J Am Coll Cardiol. 2010;55:2413-2426.
8. Massel DR, Little SH. Antiplatelet and anticoagulation for patients with prosthetic heart valves. Cochrane Database Syst Rev. 2013;7:CD003464. doi: 10.1002/14651858.CD003464.pub2.
9. Clark MA, Finkel R, Rey JA, eds. Pharmacology. 5th ed. Baltimore, MD: Lippincott Williams & Wilkins; 2012:243-264.
10. Pradaxa (dabigatran etexilate
mesylate) capsules. Detailed view: safety labeling changes approved by
FDA Center for Drug Evaluation and Research (CDER). U.S. Food and Drug
Administration. U.S. Department of Health and Human Services.
www.fda.gov/Safety/MedWatch/SafetyInformation/ucm250657.htm. Accessed
January 16, 2014.
11. Pradaxa (dabigatran) prescribing
information. Boehringer Ingelheim Pharmaceuticals, Inc. 2014.
http://bidocs.boehringer-ingelheim.com/BIWebAccess/ViewServlet.ser?docBase=renetnt&folderPath=/Prescribing%20Information/PIs/Pradaxa/Pradaxa.pdf.
12. Epocrates.com. Epocrates Essentials. Version 4.5. Accessed September 30, 2013.
13. Blood thinner dangerous for patients
with artificial heart valves, study finds. Drugs.com.
www.drugs.com/news/blood-thinner-dangerous-patients-artificial-heart-valves-study-finds-47721.html?utm_source=ddc&utm_medium=rss&utm_campaign=rss&printable=1.
Accessed September 30, 2013.
14. Van de Werf F, Brueckmann M, Connolly
SJ, et al. A comparison of dabigatran etexilate with warfarin in
patients with mechanical heart valves: the randomized, phase II study to
evaluate the safety and pharmacokinetics of oral dabigatran etexilate
in patients after heart valve replacement (RE-ALIGN). Am Heart J. 2012;163:931-937.e1.
15. Semla TP, Beizer JL, Higbee MD. Geriatric Dosage Handbook. 17th ed. Hudson, OH: Lexi-Comp Inc; 2012:912.
16. Nishimura RA, Grantham JA, Connolly
HM, et al. Low-output, low-gradient aortic stenosis in patients with
depressed left ventricular systolic function: the clinical utility of
the dobutamine challenge in the catheterization laboratory. Circulation. 2002;106:809-813.
17. Bonow RO, Carabello BA, Chatterjee K,
et al. 2008 focused update incorporated into the ACC/AHA 2006
guidelines for the management of patients with valvular heart disease: a
report of the American College of Cardiology/American Heart Association
Task Force on Practice Guidelines (Writing Committee to revise the 1998
guidelines for the management of patients with valvular heart disease).
Endorsed by the Society of Cardiovascular Anesthesiologists, Society
for Cardiovascular Angiography and Interventions, and Society of
Thoracic Surgeons. J Am Coll Cardiol. 2008;52:e1-e142.
18. Murday AJ, Hochstitzky A, Mansfield
J, et al. A prospective controlled trial of St. Jude versus Starr
Edwards aortic and mitral valve prostheses. Ann Thorac Surg. 2003;76:66-73; discussion 73-74.
19. Bryan AJ, Rogers CA, Bayliss K, et
al. Prospective randomized comparison of CarboMedics and St. Jude
Medical bileaflet mechanical heart valve prostheses: ten-year follow-up.
J Thorac Cardiovasc Surg. 2007;133:614-622.
20. Hammermeister K, Sethi GK, Henderson
WG, et al. Outcomes 15 years after valve replacement with a mechanical
versus a bioprosthetic valve: final report of the Veterans Affairs
randomized trial. J Am Coll Cardiol. 2000;36:1152-1158.
21. Oxenham H, Bloomfield P, Wheatley DJ,
et al. Twenty-year comparison of a Bjork-Shiley mechanical heart valve
with porcine bioprostheses. Heart. 2003;89:715-721.
To comment on this article, contact rdavidson@uspharmacist.com.





