US Pharm. 2007;32(6):54-61.
Glaucoma is the leading cause of irreversible blindness in the world. It is characterized by death of the retinal ganglion cells, specific visual field deficits, and optic neuropathy.1 When sufficient axonal loss occurs, peripheral vision declines, followed much later by loss of central vision.2 Most forms of glaucoma are painless, and the loss of vision is insidious.2 Although elevated intraocular pressure (IOP) is not necessary for the development of glaucoma, it is nonetheless one of the strongest risk factors along with age progression. In some individuals, glaucoma develops despite IOPs in the normal range (10-21 mmHg).3 This condition is referred to as low-tension glaucoma. On the contrary, many individuals with IOP exceeding 21 mmHg do not develop glaucoma, a condition known as ocular hypertension . There are several types of glaucoma; however, the two most common are primary open-angle glaucoma (POAG), characterized by a slow and insidious onset, and angle-closure glaucoma (ACG), which is less common and tends to be more acute. Glaucoma is further subdivided into primary (cause of outflow resistance or angle closure is unknown) and secondary (outflow resistance results from another disorder).4
Epidemiology
Glaucoma affects more than 2.2 million Americans ages 40 and older, or about
1.9% of the U.S. population.5 In general, glaucoma is more common
in African Americans and Hispanics, and with increasing age.5 In
the 65 to 69 age-group, the prevalence rate for white females is about 1.6%,
while in African American females, the rate is three times higher at 4.8%. The
disease affects more than 10% of African American men and Hispanic women over
the age of 80.5 Glaucoma appears to be more common initially in
women, but by age 65, prevalence becomes more comparable between the sexes.
5
Etiology
Anatomical and functional changes occur in patients with glaucoma. The
characteristic anatomical changes occur in the optic nerve (which contains
approximately one million axons). Convergence of axons arising from ganglion
cells of the retina takes place at the optic disc (also known as the optic
nerve head), forming a depression in the disc leading to excavation, or
cupping of the ophthalmoscopically visible optic nerve head. An increase in
axonal degeneration leads to an increase in the cup size; therefore, the
severity of glaucoma is assessed using a cup-to-disc ratio. Disc cupping is
primarily utilized to identify glaucomatous optic neuropathy versus other
optic neuropathies.
Functional changes that occur in glaucoma include progression in the deterioration of the visual field. Only small changes in decibels of visual loss are associated with ganglion cell losses of less than approximately 50%, whereas for more advanced glaucoma the visual defects increase more systematically with ganglion cell loss.6 Loss of color sensitivity, temporal contrast sensitivity, spatial resolution, and motion detection are among other functional changes. These changes occur much before the visual field defects can be seen on standard perimetry.7,8 The risk of glaucomatous damage increases with increasing IOP (especially above 22 mmHg).9
The ciliary body produces clear, aqueous humor that nourishes and circulates around the lens and cornea. Flowing out of the anterior chamber through the pupil, the aqueous humor drains primarily into the trabecular meshwork and the canal of Schlemm, and then enters the venous system. Elevated IOP occurs due to inadequate aqueous humor outflow. Although elevated IOP is strongly associated with glaucoma, it is not the sole diagnostic criterion. Some individuals experience glaucomatous optic neuropathy despite normal IOP, and, conversely, others show no signs of glaucomatous damage with IOP above normal (i.e., >21 mmHg); these individuals are diagnosed with ocular hypertension. Patients with ocular hypertension who are thought to be at moderate or high risk of developing POAG are usually given treatment for glaucoma.9 Most individuals with high IOP, however, do not have glaucomatous visual loss.10,11
Clinical Presentation
Glaucoma, also known as the "silent thief" of sight, rarely causes symptoms
until optic-nerve-fiber damages occur, creating scotomas. Clinical features
vary with the form of glaucoma. The following are the most common features
seen in most forms of glaucoma.
Elevated IOP: Pressure and severity of glaucomatous damage determines the rate at which elevated IOP causes optic nerve damage. In general, pressures of 20 to 30 mmHg usually cause damage over several years, but pressures of 40 to 50 mmHg can cause rapid visual loss and also precipitate retinovascular occlusion.12
Rainbow-Colored Rings or Halos Perceived Around Lights and Cloudy Cornea: Endothelial cells continuously remove fluid, keeping the cornea transparent. When the pressure rises quickly (ACG), the cornea becomes waterlogged, causing a fall in visual acuity and creating halos around lights.12
Pain and Redness: Rapid increase in pressure to very high levels leads to eye pain and redness. This condition is seen mostly in ACG. Nonetheless, pain is not characteristically a feature of POAG.12
Blurred Vision/Visual Field Loss: occurs as a result of damages to the retinal nerve fibers leading to arcuate scotoma, an inferior nerve-fiber-bundle defect. However, central vision is spared initially and the patient does not notice the defect. Vision may still be 6/6 even at the terminal stage of glaucomatous field loss (tunnel vision).12
Other clinical presentations that have been observed in association with the different forms of glaucoma due to the rapid buildup of IOP include nausea and vomiting, headaches, photophobia, blepharospasm (involuntary blinking or spasm of the eyelids), strabismus (misalignment of the eyes), epiphoria (excessive tearing), and amblyopia (lazy eye).
Diagnosis
Individuals at a higher risk for developing glaucoma should undergo a
comprehensive eye exam and diagnostic glaucoma test. The visual acuity test
measures vision ability at various distances utilizing an eye chart with
letters and images. Tonometry, a test performed to measure the IOP, and
ophthalmoscopy, utilized to examine the inner part of the eye (retina, optic
nerve, and blood vessels), are two routine tests performed for regular
glaucoma checkups. Pressure or optic nerve abnormalities revealed by these
examinations require additional glaucoma exams with gonioscopy and perimetry.
Gonioscopy examines the structure of the eye, determining whether the drainage
angle is closed or open. Perimetry measures all areas of vision, including
peripheral vision, "mapping out" the blind spots caused by glaucoma.
New methods--including confocal scanning laser ophthalmoscopy (HRT II), optical coherence tomography (OCT), and scanning laser polarimetry (GDx)--have been developed to provide real-time, quantitative information describing the optic disc and retinal nerve fiber layer (RNFL).13 The HRT II scans the retinal surface and optic nerve with a laser and is utilized in optic-nerve topographic (3-D) image assessment. The OCT machine can create a contour map of the optic nerve and optic cup and measure the retinal nerve fiber thickness. 14 The GDx device measures the thickness of the RNFL. These methods provide a more objective analysis of structural losses than past techniques.
Pharmacologic Treatments
The first step in therapy is to ensure that the patient abstains from
medications that may exacerbate glaucoma. These include potent
corticosteroids, which decrease the outflow of the aqueous humor, causing
irreversible eye damage and leading to POAG; anticholinergics such as atropine
and scopolamine, which precipitate ACG; and antihistamines, which increase the
pressure within the eye.
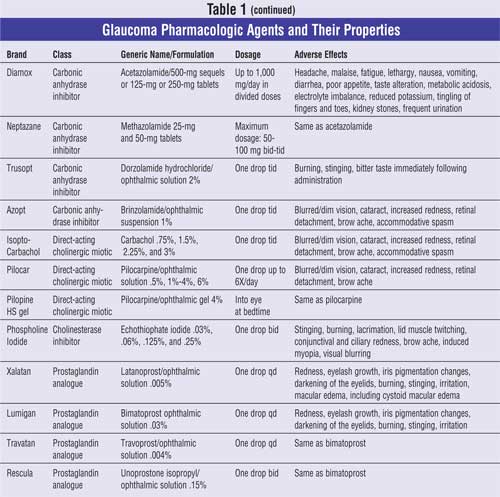
Glaucoma treatment entails reducing IOP by improving aqueous outflow, decreasing aqueous humor production, or a combination of the two. Various beta-adrenergic antagonists, adrenergic agonists, and carbonic anhydrase inhibitors aid in the reduction of aqueous humor production through various mechanisms. Miotics and prostaglandin analogues facilitate the outflow of the aqueous humor. The properties of these pharmacologic agents are described in Table 1.
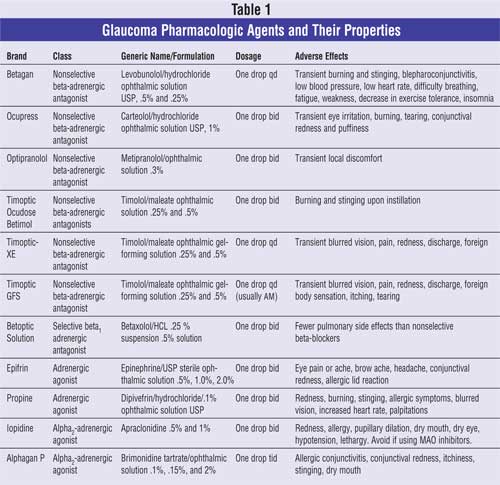
Beta-Adrenergic Antagonists: Topical beta-blockers reduce the IOP by blocking sympathetic nerve endings in the ciliary epithelium, causing a decrease in aqueous humor production. In addition, there is a possibility that the beta-blockers alter the blood flow within the ciliary processes, reducing the hydrostatic pressure within the ciliary vasculatures.15 Two types of topical beta-blockers are available for use in glaucoma: nonselective, which block both beta1 -adrenoceptors and beta2-adrenoceptors, and cardioselective, which block only beta1-receptors.
Timolol is a nonselective, beta-adrenergic receptor antagonist that does not demonstrate appreciable intrinsic sympathomimetic or membrane-stabilizing activities. It possesses a relatively high degree of lipid solubility and is subject to first-pass metabolism by the liver. Timolol is the first agent to be marketed and remains the gold standard against which all new glaucoma treatments are compared. It is available for ophthalmic use in two different formulations: sterile and gel-forming ophthalmic solutions. In addition, it may be used as an adjunctive treatment. In a multicenter, randomized, crossover comparison study it was concluded that once-daily beta-blocker therapy is an effective ocular hypotensive adjunctive treatment 24 hours after dosing when added to latanoprost.16 The baseline IOP after one month of latanoprost treatment was 20.8. Following six weeks of timolol hemihydrate treatment, the 24-hour trough IOP was 17.5, and for timolol maleate gel it was 17.9. No differences were observed between treatments in visual acuity, anterior segment findings, or adverse events. 16 Therefore, both timolol hemihydrate sterile solution and timolol maleate gel appear to be equally safe and effective.
Levobunolol, caretolol, and metipranolol are nonselective beta-adrenergic antagonists and, therefore, share the same properties as timolol. Levobunolol and timolol maleate, however, are given once daily versus twice daily.
Betaxolol is a selective beta1 -adrenergic antagonist. The exact mechanism of action is not known, but its IOP-lowering effect (which is less potent than that of the nonselective agents) is possibly mediated by a neuroprotective effect on retinal ganglionic cells.17
Although all beta-adrenergic antagonists share similar adverse effect profiles, beta2-selective agents are associated more with respiratory symptoms, just as beta1-agents are associated with cardiovascular effects such as hypotension and bradycardia. Topical beta-blockers for glaucoma or ocular hypertension may lead to new airway obstructions, requiring treatment in a population not considered to be at excess risk.18 As with other topically applied ophthalmic drugs, these agents may be absorbed systemically; however, nasolacrimal occlusion may aid with the reduction of their systemic absorption.
Adrenergic Agonists: Nonselective adrenergic agonist agents stimulate both alpha-adrenergic and beta-adrenergic receptors versus selective adrenergic agonists, which stimulate only the beta-receptor. Both subclasses are effective in reducing IOP. Alpha1 -adrenergic receptor stimulation in the ciliary body causes vasoconstriction, leading to a decrease in aqueous humor production, while stimulation of the beta2-adrenergic receptor in the trabecular meshwork and alpha2 -receptor, which control uveoscleral outflow, increases aqueous humor outflow.
Adrenaline, the first nonselective agent to be used in glaucoma treatment, has a short half-life and causes vasodilation of the conjunctival blood vessels, resulting in eye redness. Systemic side effects have also been noted with the use of adrenaline eye drops.19 Adrenaline is photosensitive, and administration of drops may lead to conjunctival pigmentation, which in extreme cases may accumulate as lachrymal stones.20 Adrenaline has also been reported to cause cystoid macular edema in patients with aphakia.21
Dipivefrin, a prodrug of epinephrine, has improved lipophilicity, achieving better corneal penetration. Due to the incidence of hyperemia of the conjuctiva, it is not generally used.
Apraclonidine, a selective alpha2 -agonist, decreases aqueous humor secretion and episcleral venous pressure. 22 It is employed to prevent or blunt the acute IOP rise after ocular laser therapy.22 It is not recommended as long-term therapy due to its high incidence of local adverse reactions and tachyphylaxis.22
Brimonidine, a selective alpha2 -agonist, is a first-line therapy used in patients who have contraindications to beta-blockers. It is also used as an adjunctive therapy when a single agent does not achieve a target IOP. In a two-identical, 12-month, randomized, double-masked multicenter trial involving 1,159 patients, a twice-daily brimonidine-timolol combination versus monotherapy with timolol or brimonidine was compared in evaluating IOP-lowering efficacy and safety.23 The mean decrease from baseline IOP during 12-month follow-up was 4.4 to 7.6 mmHg with fixed brimonidine-timolol, 2.7 to 5.5 mmHg with brimonidine, and 3.9 to 6.2 mmHg with timolol.23 Mean IOP reductions were significantly greater with fixed brimonidine-timolol compared with timolol and brimonidine. 23 The incidence of adverse events in the combination group was lower than that in the brimonidine group, but higher than that in the timolol group. The rate of discontinuation for adverse events was 14.3% with the combination, 30.6% with brimonidine, and 5.1% with timolol.23 Although the combination therapy seemed to be less tolerated when compared to the monotherapy with timolol, it provided sustained IOP lowering superior to either agent used alone.
Carbonic Anhydrase Inhibitors: Carbonic anhydrase inhibitors (CAIs) decrease the formation of bicarbonate, which is responsible for the movement of sodium and water into the eye to form aqueous humor, within the ciliary body. This mechanism leads to the reduction in aqueous humor production and, consequently, to a decrease in IOP. Acetazolamide and methazolamide are given orally due to their inability to easily penetrate the eye. This route leads to the inhibition of CA isoenzymes present in tissues other than the eye, resulting in various systemic side effects listed in Table 1. Dorzolamide and brinzolamide are used topically. Dorzolamide is used as an adjunct to beta-blockers; Cosopt is a combination of dorzolamide and timolol. A randomized, double-masked, multicenter, active-controlled, parallel group study of 215 patients with POAG or IOP was conducted to evaluate the safety and efficacy of dorzolamide versus acetazolamide when added to once-daily .5% timolol.24 Systemic adverse events were statistically greater in the acetazolamide group (75%) versus dorzolamide (50%). In addition, adverse events associated with CAI therapy were higher in the acetazolamide group (53%) versus the dorzolamide group (26%); discontinuations due to CAI adverse experiences were lower in the dorzolamide group (8%) versus acetazolamide (24%). This indicates that there is a greater incidence of systemic and CAI adverse experiences and discontinuations with oral acetazolamide compared to topical dorzolamide. 24
Direct-Acting Cholinergic Miotics: Miotics facilitate the outflow of aqueous humor from the anterior chamber of the eye by stimulation of ciliary muscles. In addition, they cause pupil constriction and "pull" on the trabecular meshwork, leading to aqueous humor outflow. However, this mechanism leads to accommodation and blurred vision, as well as other side effects listed in Table 1. Therefore, parasympathomimetic agents, most commonly pilocarpine, are considered third-line treatment options.25 Miotics are also used in combination therapies. A small, prospective, multicenter, double-masked trial involving 37 patients was conducted to establish the efficacy and safety of timolol-dorzolamide fixed combination versus timolol-pilocarpine fixed combination, each given twice daily, in POAG and ocular hypertensive patients. 26 After six weeks of treatment, with a mean baseline IOP of 22.3, the IOP was 18.0 for the timolol-dorzolamide group and 17.4 for the timolol-pilocarpine group. Statistically more patients reported ocular pain and diminished vision during use of the timolol-pilocarpine combination.26 Although both combinations provided similar efficacious reduction in IOP, the adverse effects experienced by the timolol-pilocarpine group confirm their use as third-line treatment options.
Prostaglandin Analogues: Prostaglandin analogues achieve 30% to 35% of IOP reduction by increasing aqueous outflow from the eye through the uveoscleral pathway. This drug class offers an alternative for patients who do not achieve control with another topical antiglaucoma agent or for those with a contra-indication to first-line therapy with beta-adrenergic antagonists.27 Based on preliminary clinical data, bimatoprost, iatanoprost, and travoprost appear to be at least as effective as timolol, while the effectiveness of unoprostone is similar or slightly less.27 Moreover, these drugs may be used in combination with other antiglaucoma agents to achieve better IOP control. Various studies have been conducted supporting the efficacy and safety of latanoprost as a monotherapy or in fixed combination. In a multicenter, randomized, observer-masked, six-week study involving 237 patients, latanoprost as a once-daily monotherapy was compared with a timolol-pilocarpine twice-daily combination.28 After six weeks of treatment, mean diurnal IOP in the latanoprost group was reduced by 5.4 mm Hg versus 4.9 mmHg in the timolol-pilocarpine group, a statistically significant difference. In addition, latanoprost was better tolerated than timolol-pilocarpine with regard to adverse events. These results indicate that a switch to latanoprost monotherapy can be attempted before combination therapy is initiated.28
Latanoprost, bimatoprost, and travoprost appear to be equivalent to the current standard of therapy in the topical treatment of elevated IOP.27 However, various studies demonstrate that latanoprost achieves better efficacy when compared to other agents among this drug class. It is the most powerful drug in clinical use today, and the once-daily dosing promotes compliance.29
Frequent adverse events associated with this class are hypertrichotic, conjunctiva hyperemia (engorgement of the conjunctival blood vessels), and increased iris pigmentation, which is due to increased synthesis of melanin in the melanocytes of the iris stroma. These adverse events are reversible with the exception of increased iris pigmentation. One of the initial concerns about this side effect was that melanomas would develop, but as of yet no pathologic changes have been observed in iridial melanocytes as a result of treatment with prostaglandin analogues.30 Numerous clinical studies suggest that discontinuing treatment with prostaglandin analogues on account of their side effects is rare in clinical practice.31
Conclusion
Glaucoma is a common eye disease that is usually associated with elevated IOP.
Although no medical treatments are currently available that will cure the
disease, medications, several forms of laser therapies, and incisional
surgeries aid with maintaining normal IOP and minimizing the risk of further
optic nerve damage and vision loss. Health care practitioners must carefully
weigh risks versus benefits when selecting treatment options to provide
optimal therapeutic outcomes while maintaining minimal adverse events.
References
1. Hitchings RA. Glaucoma--Fundamentals of Clinical Ophthalmology. British Med
J Publishing Group London; 2000; 222.
2. Wallace L.M. Medical management of glaucoma. N Engl J Med. 1998;339:
1298-1307.
3. Comparison of glaucomatous progression between untreated patients with
normal- tension glaucoma and patients with therapeutically reduced
Intra-ocular pressures. Collaborative Normal-Tension Glaucoma Study Group.
Am J Ophthalmol. 1998;126:487–505.
4. World Health Organization. Prevention of Blindness and Visual Impairment.
Priority eye diseases. Available at:
http://www.who.int/blindness/causes/priority/en/index7.html. Accessed December
14, 2007.
5. National Eye Institute. Vision problems in the US. General Information.
Available at: http://www.nei.nih.gov/eyedata/pdf/VPUS.pdf. Accessed December
18, 2007.
6. Harwerth RS. Ganglion cell loss underlying visual field defects from
experimental glaucoma. Investigative Ophthalmology and Visual Science.
1999;40:2242-2250.
7. Baez KA, McNaught Al, Dowler JG, et al. Motion detection threshold and
field progression in normal tension glaucoma. British J Ophthalmol.
1995;79:125-128.
8. Johnson CA, Adams AJ, Casson EF, et al. Blue-on-yelow perimetry can predict
the development of glaucomatous visual field loss. Arch Ophthalmol.
1993;111:645-650.
9. Sommer A, Tielsch JM, Katz J, et al. Relationship between Intra-ocular
pressure and primary open-angle glaucoma among white and black Americans.
Arch Ophthalmol. 1991;109:1090-1095.
10. Kass MA, Heuer DK, Higginbotham EJ, et al. The Ocular Hypertension
Treatment Study: a randomized trial determines that topical ocular hypotensive
medication delays or prevents the onset of primary open-angle glaucoma.
Arch Ophthalmol. 2002;120:701–713.
11. Leske MC. The epidemiology of open-angle glaucoma: a review. Am J
Epidemiol. 1983;118:166-191.
12. PT Khaw, P Shah, AR Elkington. Glaucoma- 1: Diagnosis. BMJ.
2004;328:97-99.
13. Sanchez-Galeana C, Bowd C, Blumenthal EZ, et al. Using optical imaging
summary data to detect glaucoma. Ophthalmology. 2001;108:1812–1818.
14. Glaucoma Research Foundation. Diagnostic Tests: General information.
Available at: http://www.glaucoma.org/learn/diagnostic_test.html. Accessed
April 4, 2007.
15 Bartlett JD, Jannus SD. Clinical Ocular Pharmacology. Maryland.
Butterworth Publishers; 1984.
16. Stewart WC, Day DG, Sharpe ED, et al. Efficacy and safety of timolol
solution once daily vs timolol gel added to latanoprost. 1999;128:692-696.
17. Wood JP, Schmidt KG, Chidlow G, et al. The beta adrenoceptor antagonists
metipranolol and timolol are retinal neuroprotectants: comparison with
betaxolol. Experimental Eye Research. 2003:76;505-516.
18. Kirwan JF, Nightingale JA, Bunce C, et al. ‚Beta-blockers for glaucoma and
excess risk of airways obstruction: population based cohort study. BMJ.
2002;325:1396-1397.
19. Delaey JJ. Systemic toxicity of sympathomimetic and parasympathomimetic
eye drops. Bulletin de la Societe Belge d'Ophthalmologie.
1979;186:21-25.
20. Bradbury JA, Rennie IG, Parsons MA. Adrenaline dacryolith: detection by
ultrasound examination of the nasolacrimal duct. British J Ophthalmol.
1988;72:935-937.
21. Classe JG, Epinepherine maculopathy. J American Optometric Association
. 1980;51:1091-1093.
22. Apatachioae I, Chiselita D. Alpha-2 adrenergic agonists in the treatment
of glaucoma. Oftalmologia. 1999;47:35-40.
23. Sherwood MB, Craven ER, Chou C, et al. Twice-daily 0.2% brimonidine-0.5%
timolol fixed-combination therapy vs monotherapy with timolol or brimonidine
in patients with glaucoma or ocular hypertension: a 12-month randomized trial.
Arch Ophthalmol. 2006;124:1230-1238.
24. Stewart WC, Halper LK, Johnson-Pratt L, et al. Tolerability and efficacy
of dorzolamide versus acetazolamide added to timolol. J Ocul Pharmacol Ther.
2002;18:211-220.
25. Lee DA, Higginbotham EJ. Glaucoma and its treatment: a review. Am. J.
Health Syst. Pharm. 2005;62:691-699.
26. JJ Kaluzny, J Szaflik, K Czechowicz-Janicka, Timolol 0.5%/dorzolamide 2%
fixed combination versus timolol 0.5%/pilocarpine 2% fixed combination in
primary open-angle glaucoma or ocular hypertensive patients. Klin Oczna
, Jan 2004;106:241-242.
27. CL Alexander, SJ Miller, and SR Abel. Prostaglandin analog treatment of
glaucoma and ocular hypertension. Annals of Pharmacotherapy.
2002;36:504-511.
28. Nordmann J-P, Söderström M, Rouland J-F, et al. Comparison of the
Intra-ocular pressure lowering effect of latanoprost and a fixed combination
of timolol-pilocarpine eye drops in patients insufficiently controlled with ‚-
adrenergic antagonists. Br J Ophthalmol. 2000;84:181-185.
29. Linden C. Therapeutic potential of prostaglandin analogues in glaucoma.
Expert Opin Investig Drugs. 2001;10:679-694.
30. Wistrand PJ, Stjernchantz J, Olsson K, et al. The incidence and time
course of latanoprost induced iridial pigmentation as a function of eye
colour. Survey of Ophthalmology. 1997;41:129-138.
31. G Hollo. The side effects of the prostaglandin analogues. Expert Opin
Drug Saf. 2007;6:45-52.
To comment on this article, contact editor@uspharmacist.com.






